Cancer incidence and mortality in Shanghai: rates in 2016 and trends from 2002 to 2016
Highlight box
Key findings
• Cancer incidence increased slightly, but cancer mortality decreased in both males and females from 2002 to 2016 in Shanghai.
What is known and what is new?
• The age-specific numbers and rates of incidence and mortality increased with age;
• The trends differed by gender and cancer type.
What is the implication, and what should change now?
• We will continue to carry our more tailored investigations on the main cancer types and key age groups through further data mining with an attempt to contribute to the reduction of the cancer burden.
Introduction
Malignant tumors are the leading cause of death worldwide and which are the major obstacle of preventing life expectancy (1). The Shanghai Municipal Center for Disease Control and Prevention updates cancer statistics annually in Shanghai. Here, we elucidate the cancer incidence and mortality in Shanghai in 2016 and analyze the trends from 2002 to 2016, with an attempt to inform the development and implementation of policies, studies, and projects related to tumor prevention and treatment.
Methods
Data sources and quality control
Case registration and quality control were carried out according to the Guideline for Chinese Cancer Registration (2) and the relevant requirements of the International Agency for Research on Cancer (IARC) on population-based tumor registration (3).
Case data were obtained from the population-based tumor registry management system and the cause of death registry system of the Shanghai Municipal Center for Disease Control and Prevention, which have been registering the diagnoses, deaths, and follow-up information of newly diagnosed cases of malignant tumors at all sites and benign tumors of the central nervous system (CNS) since 2002, covering all the household-registered populations in Shanghai (4). After investigation, verification, and coding, the data were stored in the databases of a self-developed tumor case registration management system, which was followed by regular data review and duplicate merging. The tumor site or name was coded using the International Classification of Diseases, 10th Revision (ICD-10), and the pathological type was coded using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3).
The demographic data were based on the information from the mid-year actual population and population composition regularly, which released by the Shanghai Public Security Bureau.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article did not require ethical approval, because it’s a study on surveillance system of notifiable diseases by law.
Statistical analysis
The date of first diagnosis or the data of death in all tumor registries in the period spanning 2002 to 2016 were exported to a Microsoft Access database, and all statistical analyses were completed by December 31, 2018, with Microsoft Excel and Joinpoint Regression Program.
The time of onset was defined as the date of the first diagnosis, while the age of onset was defined as the age of the patient at the time of first diagnosis. The highest diagnostic criteria were categorized into pathological diagnosis [morphological verification (MV)] and nonpathological diagnosis, which included clinical, surgical, imaging, and laboratory diagnoses as well as death certificate only (DCO). The number of cases or deaths, composition ratio, crude rate, age-specific rate (Asr), age-standardized rate (ASR), truncated ASR in the 35–64-year age group (TASR), and cumulative rates for the 0–64-, 0–74-, and 0–84-year age groups are presented by age of diagnosis or death, gender, age group, and cancer type. The Asrs were calculated for the various age groups, beginning from 0 to 85 years and above for a total of 18 5-year individual or combined age groups. Age-standardized and truncated rates were calculated uniformly using the 1960 Segi’s world standard population (5).
Comparisons of crude and Asrs were performed using z score, and comparisons of standardization rates were performed using the weighted chi-square (Cochran) test (the sign of the statistic is noted as X) (6). The temporal trend changes of year-by-year rates were calculated using Joinpoint Regression Program 4.8.0.1 software developed by the National Cancer Institute (NCI), which not only calculated the annual percent change (APC) in rate values over the 15-year period from 2002 to 2016 (7) but also determined the statistical significance of the differences in trend changes within different time periods through use of the Joinpoint regression model (8). Piecewise linear description of the long-term trends was performed based on the best-fit results, and the APCs of each piece (line segment), Joinpoint, and curve-fit value were obtained. The maximum number of Joinpoint was based on the length of the period, and 1 line segment was set to cover at least 5 consecutive years in this study. A t-test was applied to determine whether the difference between the APC and 0 was statistically significant and to determine whether the APC difference between two adjacent segments was statistically significant, so as to ascertain the Joinpoints.
All tests were two-tailed, and a P value of <0.05 was considered statistically significant.
Results
Overall cancer incidence and mortality rates
Overview
In 2016, the percentage of news cases with morphological verification (MV%) was 75.76% in Shanghai, the percentage of cases with DCO was 0.39%, the mortality to incidence (M/I) ratio was 0.50, and the proportion of other and unspecified sites (O&U) was 1.71%.
In 2016, there were 74,422 newly diagnosed cancer cases in Shanghai, with 38,857 (52.21%) of these being males and 35,565 (47.79%) being females. The crude rate of incidence was 513.94/100,000 (540.88/100,000 for males and 487.42/100,000 for females), and the age-standardized incidence rate was 231.58/100,000 (230.85/100,000 for males and 234.85/100,000 for females; X=1.99; P=0.047). The TASR of incidence was 397.86/100,000, with the cumulative incidence rate being 14.62% for the 0–64-year group, 25.14% for the 0–74-year group, and 41.39% for the 0–84-year group.
A total of 37,010 people died from malignant tumors in Shanghai in 2016, among whom 22,742 (61.45%) were male and 14,268 (38.55%) were female. The crude rate of mortality was 255.58/100,000 (316.56/100,000 for males and 195.55/100,000 for females), and the age-standardized mortality rate was 90.01/100,000 (117.52/100,000 for males and 64.90/100,000 for females). The TASR of mortality was 110.74/100,000, with the cumulative incidence rate being 4.02% for the 0–64-year group, 9.40% for the 0–74-year group, and 21.89% for the 0–84-year group (Table 1).
Table 1
| Category | Gender | Number | Proportion (%) | Crude rate (10−5) | ASRa (10−5) | TASRb (10−5) | Cumulative rate (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 35–64 years | 0–64 years | 0–74 years | 0–84 years | |||||||
| Incidence | Both | 74,422 | 100.00 | 513.94 | 231.58 | 397.86 | 14.62 | 25.14 | 41.39 | |
| Male | 38,857 | 52.21 | 540.88 | 230.85 | 343.25 | 12.85 | 25.72 | 47.03 | ||
| Female | 35,565 | 47.79 | 487.42 | 234.85 | 452.51 | 16.39 | 24.56 | 36.71 | ||
| Mortality | Both | 37,010 | 100.00 | 255.58 | 90.01 | 110.74 | 4.02 | 9.40 | 21.89 | |
| Male | 22,742 | 61.45 | 316.56 | 117.52 | 137.01 | 5.03 | 12.32 | 29.23 | ||
| Female | 14,268 | 38.55 | 195.55 | 64.90 | 84.54 | 3.02 | 6.46 | 15.41 | ||
a, ASR: age-standardized rate adjusted by world standard population (Segi’s 1960); b, TASR: truncated age-standardized rate of 35–64 years old adjusted by world standard population (Segi’s 1960).
By age
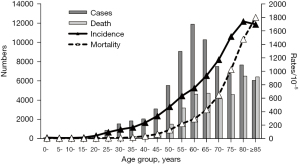
The number of age-specific cancer cases and deaths increased with age in Shanghai in 2016. The number of cases peaked in the 60–64-year group (n=11,869) and then somehow decreased; the number of deaths was the highest in the 80–84-year group (n=6,482).
The age-specific incidence and mortality rates of cancers increased with age in Shanghai in 2016, with the age-specific incidence rising from 14.65/100,000 in the 0–4-year group to 1,736.96/100,000 in the 80–84-year group, and the age-specific mortality rate rising from 4.31/100,000 in the 0–4-year group to 1,796.94/100,000 in the 85-years-and-older group (Figure 1).
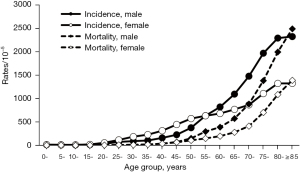
The age-specific incidence and mortality rates of cancers increased with age in both males and females in Shanghai in 2016, and the basic features were consistent with the abovementioned combined statistics. The age-specific incidence rate for females was 54.53/100,000 in the 20–24-year group, which was higher than that in males (33.16/100,000; z=3.80, P<0.001). The age-specific incidence rate in the 55–59-year group for females was 632.76/100,000, which was not significantly different from that in males (634.70/100,000; z=0.15, P=0.884). In the 60–64-year group, females again showed lower incidence rates (680.64/100,000 vs. 820.85/100,000; z=10.22, P<0.001). The age-specific mortality rate for women was 107.22/100,000 in the 50–54-year group, which was lower than that of males (149.86/100,000; z=6.42, P<0.001; Figure 2).
In the analysis of different age groups, there were 174 (0.23%) cases in children aged 0–14 years, 4,530 (6.09%) in adolescents and young adults (AYA; 15–39-year group), 19,693 (26.46%) in middle-aged adults (40–59-year group), 36,353 (48.85%) in the 60–79-year group, and 13,672 (18.37%) in the 80-year-and-older group; the number and proportions of deaths in these age groups were 59 (0.16%), 445 (1.20%), 5,613 (15.17%), 18,011 (48.67%), and 12,882 (34.81%), respectively (Table 2).
Table 2
| Age group (years) | Ranka | Incidence | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Site | Number | Proportionb (%) | Asr (10−5) | Site | Number | Proportion (%) | Asr (10−5) | |||
| 0–14 | All | 174 | 0.23 | 11.97 | All | 59 | 0.16 | 4.06 | ||
| 1 | Leukemia | 65 | 37.36 | 4.47 | Brain, CNS | 24 | 40.68 | 1.65 | ||
| 2 | Brain, CNS | 33 | 18.97 | 2.27 | Leukemia | 17 | 28.81 | 1.17 | ||
| 3 | Lymphoma | 14 | 8.05 | 0.96 | Soft tissue | 6 | 10.17 | 0.41 | ||
| 4 | Soft tissuec | 12 | 6.90 | 0.83 | Bone | 3 | 5.08 | 0.21 | ||
| 5 | Kidney | 7 | 4.02 | 0.48 | Lymphoma | 2 | 3.39 | 0.14 | ||
| 15–39 | All | 4,530 | 6.09 | 110.75 | All | 445 | 1.20 | 10.88 | ||
| 1 | Thyroid | 2,380 | 52.54 | 58.18 | Leukemia | 55 | 12.36 | 1.34 | ||
| 2 | Breast | 388 | 8.57 | 9.49 | Stomach | 44 | 9.89 | 1.08 | ||
| 3 | Lung | 291 | 6.42 | 7.11 | Liver | 38 | 8.54 | 0.93 | ||
| 4 | Brain, CNS | 159 | 3.51 | 3.89 | Colorectum | 37 | 8.31 | 0.90 | ||
| 5 | Cervix uterid | 144 | 3.18 | 7.07 | Lung | 32 | 7.19 | 0.78 | ||
| 40–59 | All | 19,693 | 26.46 | 450.12 | All | 5,613 | 15.17 | 128.29 | ||
| 1 | Thyroid | 3,556 | 18.06 | 81.28 | Lung | 1,232 | 21.95 | 28.16 | ||
| 2 | Lung | 3,309 | 16.80 | 75.63 | Liver | 759 | 13.52 | 17.35 | ||
| 3 | Breast | 2,435 | 12.36 | 55.66 | Colorectum | 519 | 9.25 | 11.86 | ||
| 4 | Colorectum | 1,835 | 9.32 | 41.94 | Stomach | 501 | 8.93 | 11.45 | ||
| 5 | Stomach | 1,178 | 5.98 | 26.93 | Pancreas | 370 | 6.59 | 8.46 | ||
| 60–79 | All | 36,353 | 48.85 | 965.30 | All | 18,011 | 48.67 | 478.26 | ||
| 1 | Lung | 8,086 | 22.24 | 214.71 | Lung | 5,055 | 28.07 | 134.23 | ||
| 2 | Colorectum | 5,178 | 14.24 | 137.49 | Colorectum | 2,006 | 11.14 | 53.27 | ||
| 3 | Stomach | 3,301 | 9.08 | 87.65 | Stomach | 1,990 | 11.05 | 52.84 | ||
| 4 | Breast | 2,333 | 6.42 | 61.95 | Liver | 1,575 | 8.74 | 41.82 | ||
| 5 | Prostatee | 2,116 | 5.82 | 113.34 | Pancreas | 1,335 | 7.41 | 35.45 | ||
| ≥80 | All | 13,672 | 18.37 | 1,717.82 | All | 12,882 | 34.81 | 1618.56 | ||
| 1 | Lung | 2,709 | 19.81 | 340.37 | Lung | 2,851 | 22.13 | 358.21 | ||
| 2 | Colorectum | 2,133 | 15.60 | 268.00 | Colorectum | 2,011 | 15.61 | 252.67 | ||
| 3 | Stomach | 1,368 | 10.01 | 171.88 | Stomach | 1,430 | 11.10 | 179.67 | ||
| 4 | Prostate | 942 | 6.89 | 295.37 | Liver | 902 | 7.00 | 113.33 | ||
| 5 | Liver | 924 | 6.76 | 116.10 | Pancreas | 837 | 6.50 | 105.16 | ||
a, sorted by the number of cases or deaths; b, the denominator of the proportions of all was the sum number of all age groups, but that of major site was the all number of each age group; c, connective and soft tissue; d, cervix uteri cancer only occurred in females, and thus this is the age-specific rate of males; e, prostate cancer only occurred in males, and thus this is the age-specific rate of females. Asr, age-specific rate; CNS, central nervous system.
Trends during in the 2002–2016 period
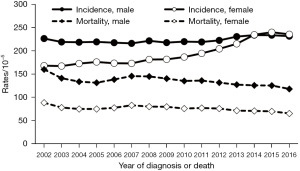
The age-standardized incidence rate of cancers in males in Shanghai rose from 225.76/100,000 in 2002 to 230.85/100,000 in 2016. No statistically significant trend was seen between with 2002 and 2009 (APC =–0.32, t=–1.09, P=0.301); that is, the age-standardized incidence rate in males was generally stable during this period; however, it grew at an average annual rate of 1.16% in the 2009–2016 period (APC =1.16, t=4.37, P=0.001). The age-standardized incidence rate for females rose from 167.44 to 234.85 per 100,000, after which it also remained stable between 2002 and 2009 (APC =1.09, t=2.08, P=0.064); it increased at an average annual rate of 4.48% between 2009 and 2016 (APC =4.48, t=9.67, P=0.001), rising to 233.96/100,000 in 2014, but this was not significantly different from that for males (233.14/100,000) in the same year (X=0.40, P=0.687); it rose to 239.30/100,000 in 2015, which was the first time it was higher than the male rate (232.92/100,000; X=3.10, P=0.002).
The age-standardized mortality rate of cancers in males in Shanghai changed from 159.2/100,000 to 117.52/100,000 in the 2002–2016 period, representing a decreasing trend with an average annual decline of 1.35% over 15 years (APC =−1.35, t=–4.87, P<0.001). The age-standardized mortality rate of cancers in females in Shanghai declined from 87.91/100,000 to 64.90/100,000 in the 2002–2016 period, and the average annual decline rate was 1.31% over 15 years (APC =–1.31, t=–4.53, P<0.001) (Figure 3).
Incidence and mortality rates of common cancers
Overview
Lung cancer was the most common cancer in Shanghai in 2016, both in terms of incidence and mortality. There were 14,395 new cases, accounting for 19.34% of all malignant tumor cases. The crude rate of incidence was 99.41/100,000, and the age-standardized incidence rate was 39.76/100,000. In all, 9,170 people died of lung cancer, accounting for 24.78% of all malignant tumor-related deaths, with a crude mortality rate of 63.33/100,000 and an age-standardized mortality rate of 21.57/100,000. The incidence and mortality ranked first in both males and females.
Colorectal cancer was the second most common malignancy in terms of both incidence and mortality; it ranked second in both incidence and mortality in males, and ranked second and fourth in mortality and incidence in females, respectively.
Thyroid cancer was the third most common malignant tumor, with 7,683 cases newly diagnosed in Shanghai in 2016; however, only 138 deaths due to thyroid cancer were reported in Shanghai in 2016, suggesting thyroid cancer is not a common cause of cancer death. Thyroid cancer ranked sixth in incidence among males and third among females, and its mortality rate was outside the top 10 for both males and females.
Stomach cancer was the fourth most common malignancy, followed by breast cancer, liver cancer, prostate cancer, pancreatic cancer, brain and other CNS tumors, and bladder cancer. The above top 10 cancers accounted for 76.15% of all malignant tumors.
Stomach cancer ranked third in terms of number of deaths, followed by liver cancer, pancreatic cancer, breast cancer, gallbladder cancer, esophageal cancer, prostate cancer, and lymphoma. The case number of the above top 10 cancers accounted for 78.85% of all malignant tumors.
Stratified analyses by gender showed that prostate cancer ranked fourth in incidence and sixth in mortality in males; breast cancer ranked second in incidence and fourth in mortality in females, with only 32 cases and 11 deaths among males; cervical cancer ranked ninth in incidence and tenth in mortality in females; ovarian cancer ranked tenth in incidence and eighth in mortality in females.
Comparisons of the age-standardized incidence rates of various common cancers between males and females showed that the age-standardized incidence rate of thyroid cancer was 21.98/100,000 for males, which was significantly lower than that in females (54.28/100,000; X=33.20, P<0.001); the age-standardized incidence rate of brain and other CNS tumors was 6.40/100,000 in males, which was significantly lower than that in females (7.63/100,000; X=2.89, P=0.004); the age-standardized incidence rate of gallbladder cancer was 3.04/100,000 in males, which was also significantly lower than that in females (3.78/100,000; X=3.75, P<0.001); however, the age-standardized incidence rates of lung, colorectal, gastric, liver, pancreatic, bladder, kidney, and esophageal cancers were all higher in males than in females. Comparisons of age-standardized mortality rates showed that the age-standardized mortality rate of gallbladder cancer was 2.37/100,000 in males, which was significantly lower than in females (3.03/100,000; X=3.85, P<0.001), whereas the age-standardized mortality rates of lung, colorectal, gastric, liver, pancreatic, esophageal, and bladder cancers, lymphoma, and brain and CNS tumors were all higher in males than in females (Table 3).
Table 3
| Category | Ranka | Both | Male | Female | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | Number | Proportion (%) | Crude rate (10−5) | ASR (10−5) | Site | Number | Proportion (%) | Crude rate (10−5) | ASR (10−5) | Site | Number | Proportion (%) | Crude rate (10−5) | ASR (10−5) | ||||
| Incidence | 1 | Lung | 14,395 | 19.34 | 99.41 | 39.76 | Lung | 8,786 | 22.61 | 122.30 | 47.46 | Lung | 5,609 | 15.77 | 76.87 | 32.82 | ||
| 2 | Colorectum | 9,285 | 12.48 | 64.12 | 24.52 | Colorectum | 5,412 | 13.93 | 75.33 | 29.88 | Breast | 5,584 | 15.70 | 76.53 | 39.69 | |||
| 3 | Thyroid | 7,683 | 10.32 | 53.06 | 38.10 | Stomach | 3,933 | 10.12 | 54.75 | 21.19 | Thyroid | 5,580 | 15.69 | 76.47 | 54.28 | |||
| 4 | Stomach | 5,979 | 8.03 | 41.29 | 15.85 | Prostate | 3,226 | 8.30 | 44.91 | 15.47 | Colorectum | 3,873 | 10.89 | 53.08 | 19.42 | |||
| 5 | Breast | 5,616 | 7.55 | 38.78 | 20.03 | Liver | 2,668 | 6.87 | 37.14 | 15.87 | Stomach | 2,046 | 5.75 | 28.04 | 10.84 | |||
| 6 | Liver | 3,842 | 5.16 | 26.53 | 10.60 | Thyroid | 2,103 | 5.41 | 29.27 | 21.98 | Pancreas | 1,349 | 3.79 | 18.49 | 6.03 | |||
| 7 | Prostateb | 3,226 | 4.33 | 44.91 | 15.47 | Pancreas | 1,594 | 4.10 | 22.19 | 8.56 | Liver | 1,174 | 3.30 | 16.09 | 5.45 | |||
| 8 | Pancreas | 2,943 | 3.95 | 20.32 | 7.28 | Bladder | 1,437 | 3.70 | 20.00 | 7.43 | Brain, CNS | 1,080 | 3.04 | 14.80 | 7.63 | |||
| 9 | Brain, CNS | 1,895 | 2.55 | 13.09 | 7.02 | Kidney | 1,101 | 2.83 | 15.33 | 7.22 | Cervix uteri | 987 | 2.78 | 13.53 | 8.26 | |||
| 10 | Bladder | 1,808 | 2.43 | 12.49 | 4.46 | Esophagus | 1,100 | 2.83 | 15.31 | 5.74 | Gallbladder | 837 | 2.35 | 11.47 | 3.78 | |||
| Mortality | 1 | Lung | 9,170 | 24.78 | 63.33 | 21.57 | Lung | 6,622 | 29.12 | 92.18 | 33.20 | Lung | 2,548 | 17.86 | 34.92 | 10.77 | ||
| 2 | Colorectum | 4,573 | 12.36 | 31.58 | 10.07 | Colorectum | 2,667 | 11.73 | 37.12 | 12.86 | Colorectum | 1,906 | 13.36 | 26.12 | 7.59 | |||
| 3 | Stomach | 3,965 | 10.71 | 27.38 | 9.32 | Stomach | 2,642 | 11.62 | 36.78 | 12.96 | Stomach | 1,323 | 9.27 | 18.13 | 6.05 | |||
| 4 | Liver | 3,275 | 8.85 | 22.62 | 8.65 | Liver | 2,274 | 10.00 | 31.65 | 13.05 | Breast | 1,306 | 9.15 | 17.90 | 6.79 | |||
| 5 | Pancreas | 2,554 | 6.90 | 17.64 | 6.01 | Pancreas | 1,364 | 6.00 | 18.99 | 7.05 | Pancreas | 1,190 | 8.34 | 16.31 | 4.98 | |||
| 6 | Breast | 1,317 | 3.56 | 9.09 | 3.51 | Prostate | 1,067 | 4.69 | 14.85 | 4.34 | Liver | 1,001 | 7.02 | 13.72 | 4.40 | |||
| 7 | Gallbladder | 1,204 | 3.25 | 8.31 | 2.72 | Esophagus | 920 | 4.05 | 12.81 | 4.63 | Gallbladder | 721 | 5.05 | 9.88 | 3.03 | |||
| 8 | Esophagus | 1,171 | 3.16 | 8.09 | 2.67 | Bladder | 579 | 2.55 | 8.06 | 2.50 | Ovary | 419 | 2.94 | 5.74 | 2.44 | |||
| 9 | Prostate | 1,067 | 2.88 | 18.99 | 7.05 | Lymphoma | 524 | 2.30 | 7.29 | 3.07 | Brain, CNS | 415 | 2.91 | 5.69 | 2.54 | |||
| 10 | Lymphoma | 888 | 2.40 | 6.13 | 2.46 | Gallbladder | 483 | 2.12 | 6.72 | 2.37 | Lymphoma | 364 | 2.55 | 4.99 | 1.88 | |||
a, sorted by the number of cases or deaths; b, prostate cancer only occurred in males, and thus this is the age-specific rate of females. ASR, age-standardized rate; CNS, central nervous system.
By age
Stratification by the common age groups showed that leukemia had the highest proportion (37.36%, n=65) of cancer incidence among children aged 0–14 years in Shanghai in 2016, followed by brain and other CNS tumors, lymphoma, soft tissue malignant tumors, and kidney cancer; brain and other CNS tumors ranked first in mortality, with a proportion of 40.68% (n=24), followed by leukemia, soft tissue malignant tumors, bone cancer, and lymphoma.
In the 15–39-year group, thyroid cancer ranked first in incidence, with a proportion of 52.54% (n=2,380), followed by breast cancer, lung cancer, brain and other CNS tumors, and cervical cancer; leukemia ranked first in mortality, with a proportion of 12.36% (n=55), followed by stomach cancer, liver cancer, colorectal cancer, and lung cancer.
In the 40–59-year group, thyroid cancer ranked first in incidence, with a proportion of 18.06% (n=3,556), followed by lung cancer, breast cancer, colorectal cancer, and stomach cancer; lung cancer ranked first in mortality, with a proportion of 21.95% (n=1,232), followed by liver cancer, colorectal cancer, stomach cancer, and pancreatic cancer.
In the 60–79-year group, lung cancer ranked first in incidence, with a proportion of 22.24% (n=8,086), followed by colorectal cancer, stomach cancer, breast cancer, and prostate cancer; lung cancer ranked first in mortality, with a proportion of 28.07% (n=5,055), followed by colorectal cancer, stomach cancer, liver cancer, and pancreatic cancer.
In the 80-years-and-older age group, lung cancer ranked first in incidence, with a proportion of 19.81% (n=2709), followed by colorectal cancer, gastric cancer, prostate cancer, and liver cancer; lung cancer ranked first in mortality, with a proportion of 22.13% (n=2,851), followed by colorectal cancer, stomach cancer, liver cancer, and pancreatic cancer.
The combined numbers of both cases and deaths of the top 5 cancers in all age groups exceeded 50%, except for the 15–39-years group, where the combined number of deaths of the top 5 tumors was 46.29% (Table 2).
Trends during from 2002 to 2016
A total of 16 cancers were found to be among the top 10 tumors in terms of incidence and mortality in Shanghai in 2016. The trends of the change in the age-standardized incidence and mortality rates for the top 10 tumors in terms of incidence and mortality in males and females in Shanghai in 2016 are detailed in Tables 4,5.
Table 4
| Gender | Site | Year of 2002 | Joinpoint | Year of 2016 | Trend 1 | Trend 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASRa (10−5) | Year | ASR (10−5) | ASR (10−5) | Years | APCb | t | P value | Years | APC | t | P value | ||||||
| Male | Lung | 51.48 | 2010 | 45.39 | 47.46 | 2002–2010 | −1.35 | −3.09 | 0.011 | 2010–2016 | 1.67 | 2.74 | 0.021 | ||||
| Colorectum | 24.58 | – | – | 29.88 | 2002–2016 | 1.82 | 6.68 | <0.001 | – | – | – | – | |||||
| Thyroid | 1.72 | – | – | 21.98 | 2002–2016 | 22.59 | 22.94 | <0.001 | – | – | – | – | |||||
| Stomach | 33.25 | – | – | 21.19 | 2002–2016 | −2.94 | −20.71 | <0.001 | – | – | – | – | |||||
| Liver | 27.53 | – | – | 15.87 | 2002–2016 | −3.67 | −23.31 | <0.001 | – | – | – | – | |||||
| Prostate | 6.92 | 2010 | 12.27 | 15.47 | 2002–2010 | 7.54 | 15.85 | <0.001 | 2010–2016 | 3.91 | 8.00 | <0.001 | |||||
| Pancreas | 7.34 | – | – | 8.56 | 2002–2016 | 0.72 | 3.87 | 0.002 | – | – | – | – | |||||
| Bladder | 7.64 | – | – | 7.43 | 2002–2016 | −0.26 | −1.62 | 0.129 | – | – | – | – | |||||
| Kidney | 4.68 | – | – | 7.22 | 2002–2016 | 3.54 | 6.40 | <0.001 | – | – | – | – | |||||
| Brain, CNS | 6.63 | – | – | 6.40 | 2002–2016 | 0.05 | 0.14 | 0.892 | – | – | – | – | |||||
| Lymphoma | 5.75 | 2012 | 5.27 | 6.33 | 2002–2012 | −0.32 | −1.34 | 0.209 | 2012–2016 | 3.26 | 3.52 | 0.006 | |||||
| Esophagus | 10.62 | – | – | 5.74 | 2002–2016 | −4.20 | −26.82 | <0.001 | |||||||||
| Gallbladder | 3.17 | 2010 | 3.42 | 3.04 | 2002–2010 | 1.79 | 2.64 | 0.025 | 2010–2016 | −2.44 | −2.73 | 0.021 | |||||
| Female | Lung | 18.45 | 2010 | 18.79 | 32.82 | 2002–2010 | 0.19 | 0.22 | 0.832 | 2010–2016 | 11.31 | 10.05 | <0.001 | ||||
| Thyroid | 5.29 | – | – | 54.28 | 2002–2016 | 19.52 | 21.78 | <0.001 | – | – | – | – | |||||
| Breast | 30.75 | – | – | 39.69 | 2002–2016 | 1.90 | 9.40 | <0.001 | – | – | – | – | |||||
| Colorectum | 20.50 | – | – | 19.42 | 2002–2016 | 0.03 | 0.15 | 0.887 | – | – | – | – | |||||
| Stomach | 15.63 | – | – | 10.84 | 2002–2016 | −2.70 | −20.80 | <0.001 | – | – | – | – | |||||
| Cervix uteri | 3.18 | 2010 | 7.45 | 8.26 | 2002–2010 | 12.51 | 10.22 | <0.001 | 2010–2016 | 3.12 | 2.36 | 0.040 | |||||
| Brain, CNS | 7.84 | – | – | 7.63 | 2002–2016 | 0.27 | 1.16 | 0.269 | – | – | – | – | |||||
| Pancreas | 5.24 | – | – | 6.03 | 2002–2016 | 0.98 | 4.27 | 0.001 | – | – | – | – | |||||
| Liver | 9.51 | – | – | 5.45 | 2002–2016 | −3.88 | −23.46 | <0.001 | – | – | – | – | |||||
| Ovary | 6.53 | – | – | 5.33 | 2002–2016 | −1.78 | −4.78 | <0.001 | – | – | – | – | |||||
| Lymphoma | 3.84 | – | – | 4.50 | 2002–2016 | 0.77 | 2.00 | 0.067 | – | – | – | – | |||||
| Gallbladder | 4.58 | – | – | 3.78 | 2002–2016 | −1.56 | −4.82 | <0.001 | – | – | – | – | |||||
| Kidney | 2.07 | – | – | 3.16 | 2002–2016 | 2.95 | 4.49 | 0.001 | – | – | – | – | |||||
| Bladder | 2.02 | – | – | 1.72 | 2002–2016 | −1.01 | −2.67 | 0.019 | – | – | – | – | |||||
| Esophagus | 2.77 | – | – | 1.07 | 2002–2016 | −6.41 | −19.27 | <0.001 | – | – | – | – | |||||
a, ASR: age-standardized rate adjusted by world standard population (Segi’s 1960); b, APC: annual percent change calculated by Joinpoint Regression Program. CNS, central nervous system.
Table 5
| Gender | Site | Year of 2002 | Joinpoint | Year of 2016 | Trend 1 | Trend 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASRa (10−5) | Year | ASR (10−5) | ASR (10−5) | Years | APCb | t | P value | Years | APC | t | P value | ||||||
| Male | Lung | 43.40 | – | – | 33.20 | 2002–2016 | −1.10 | −3.81 | 0.002 | – | – | – | – | ||||
| Liver | 24.74 | – | – | 13.05 | 2002–2016 | −3.72 | −13.61 | <0.001 | – | – | – | – | |||||
| Stomach | 24.39 | – | – | 12.96 | 2002–2016 | −3.36 | −9.72 | <0.001 | – | – | – | – | |||||
| Colorectum | 13.37 | – | – | 12.86 | 2002–2016 | 0.90 | 2.37 | 0.034 | – | – | – | – | |||||
| Pancreas | 7.00 | 2009 | 7.46 | 7.05 | 2002–2009 | 2.56 | 2.42 | 0.036 | 2009–2016 | −0.78 | −0.87 | 0.403 | |||||
| Esophagus | 9.53 | – | – | 4.63 | 2002–2016 | −3.90 | −9.47 | <0.001 | – | – | – | – | |||||
| Prostate | 2.91 | 2012 | 4.87 | 4.34 | 2002–2012 | 6.11 | 9.35 | <0.001 | 2012–2016 | −2.59 | −1.45 | 0.180 | |||||
| Brain, CNS | 3.91 | – | – | 3.25 | 2002–2016 | −0.42 | −1.15 | 0.272 | – | – | – | – | |||||
| Lymphoma | 3.30 | – | – | 3.07 | 2002–2016 | −0.52 | −1.88 | 0.083 | – | – | – | – | |||||
| Bladder | 3.21 | – | – | 2.50 | 2002–2016 | −1.11 | −3.34 | 0.005 | – | – | – | – | |||||
| Gallbladder | 2.68 | 2010 | 2.89 | 2.37 | 2002–2010 | 2.58 | 2.75 | 0.020 | 2010–2016 | −2.99 | −2.45 | 0.030 | |||||
| Kidney | 1.62 | – | – | 1.68 | 2002–2016 | 2.21 | 3.36 | 0.005 | – | – | – | – | |||||
| Thyroid | 0.26 | – | – | 0.20 | 2002–2016 | −0.52 | −0.47 | 0.649 | – | – | – | – | |||||
| Female | Lung | 15.10 | – | – | 10.77 | 2002–2016 | −0.98 | −2.43 | 0.030 | – | – | – | – | ||||
| Colorectum | 10.00 | – | – | 7.59 | 2002–2016 | −0.77 | −2.20 | 0.047 | – | – | – | – | |||||
| Breast | 7.71 | 2009 | 8.05 | 6.79 | 2002–2009 | 1.92 | 2.33 | 0.042 | 2009–2016 | −2.01 | −2.75 | 0.021 | |||||
| Stomach | 10.92 | – | – | 6.05 | 2002–2016 | −3.14 | −8.08 | <0.001 | – | – | – | – | |||||
| Pancreas | 5.02 | – | – | 4.98 | 2002–2016 | 0.63 | 1.55 | 0.146 | – | – | – | – | |||||
| Liver | 8.40 | – | – | 4.40 | 2002–2016 | −4.29 | −11.97 | <0.001 | – | – | – | – | |||||
| Gallbladder | 4.01 | 2011 | 3.67 | 3.03 | 2002–2011 | 0.28 | 0.37 | 0.718 | 2011–2016 | −4.44 | −2.61 | 0.026 | |||||
| Brain, CNS | 2.99 | – | – | 2.54 | 2002–2016 | −0.59 | −2.31 | 0.038 | – | – | – | – | |||||
| Ovary | 2.89 | – | – | 2.44 | 2002–2016 | −0.28 | −0.80 | 0.441 | – | – | – | – | |||||
| Lymphoma | 1.98 | – | – | 1.88 | 2002–2016 | −0.58 | −1.34 | 0.202 | – | – | – | – | |||||
| Cervix uteri | 1.25 | – | – | 1.87 | 2002–2016 | 4.52 | 8.52 | <0.001 | – | – | – | – | |||||
| Esophagus | 2.44 | 2009 | 1.71 | 0.81 | 2002–2009 | −4.27 | −3.11 | 0.011 | 2009–2016 | −8.58 | −5.84 | <0.001 | |||||
| Kidney | 0.68 | – | – | 0.69 | 2002–2016 | 0.62 | 0.88 | 0.398 | – | – | – | – | |||||
| Bladder | 0.78 | – | – | 0.51 | 2002–2016 | −2.07 | −3.49 | 0.004 | – | – | – | – | |||||
| Thyroid | 0.57 | – | – | 0.40 | 2002–2016 | −0.04 | −0.04 | 0.968 | – | – | – | – | |||||
a, ASR: age-standardized rate adjusted by world standard population (Segi’s 1960); b, APC: annual percent change calculated by Joinpoint Regression Program. CNS, central nervous system.
The age-standardized incidence rate of lung cancer in males in Shanghai decreased from 51.48/100,000 in 2002 to 47.46/100,000 in 2016, showing a significant decline at an annual average rate of –1.35% during the 2002–2010 period (APC =–1.35, t=−3.09, P=0.011) and a significant rise at an average annual rate of 1.67% during the 2010-2016 period (APC =1.67, t=2.74, P=0.021). The age-standardized incidence rate of lung cancer in females in Shanghai rose from 18.45/100,000 in 2002 to 32.82/100,000 in 2016; it was stable during the 2002–2010 period (APC =0.19, t=0.22, P=0.832) and significantly increased at an average annual rate of 11.31% during the 2010–2016 period (APC =11.31, t=10.05, P=0.001). The age-standardized mortality rates showed a decline in both males and females.
Except for lung cancer, the age-standardized incidence rates of all examined cancers, including colorectal cancer, thyroid cancer, prostate cancer, pancreatic cancer, and kidney cancer rose in males during the 2002–2016 period; notably, the average annual growth rate of thyroid cancer reached 22.59%, and the average annual growth rate of prostate cancer was 7.54% during the 2002–2010 period and decreased to 3.91% during the 2010-2016 period. The age-standardized incidence rates of lymphoma were stable during from 2002 to 2012, but increased on average by 3.26% per year from 2012 to 2016. The age-standardized incidence rates of bladder cancer and brain and other CNS tumors remained stable during from 2002 to 2016. The age-standardized incidence rates of stomach, liver, and esophageal cancers showed decreasing trends in different degrees during from 2002 to 2016. The age-standardized incidence rates of gallbladder cancer were stable during from 2002 to 2010 but decreased on average by 2.44% per year from 2010 to 2016.
Except for lung cancer, the age-standardized incidence rates of all examined cancers, including thyroid cancer, breast cancer, cervical cancer, pancreatic cancer, and kidney cancer showed rising trends in females during in the 2002–2016 period; notably, the average annual growth rate of thyroid cancer reached 19.52%, the average annual growth rate of cervical cancer was 12.51% during the 2002–2010 period and decreased to 3.12% during the 2010–2016 period. The age-standardized incidence rates of colorectal cancer, brain and other CNS tumors, and lymphoma remained stable during from 2002 to 2016. The age-standardized incidence rates of stomach cancer, liver cancer, ovarian cancer, gallbladder cancer, bladder cancer, and esophageal cancer showed decreasing trends in different degrees during from 2002 to 2016.
Except for lung cancer, the age-standardized mortality rates of colorectal cancer and kidney cancer showed increasing trends to varying degrees during from 2002 to 2016. The age-standardized mortality rates of brain and other CNS tumors, lymphoma, and thyroid cancer remained stable during from 2002 to 2016. The age-standardized mortality rates of pancreatic cancer increased on average by 2.56% per year during from 2002 to 2009 and remained stable during the 2009–2016 period. The age-standardized mortality rates of prostate cancer increased on average by 6.11% per year during from 2002 to 2012 and remained stable during from2012 to 2016. The age-standardized mortality rates of liver, stomach, esophagus, and bladder cancers showed decreasing trends to varying degrees during the 2002–2016 period. The age-standardized mortality rates of gallbladder cancer increased on average by 2.58% per year during from 2002 to 2010 but decreased on average by 2.99% per year from 2010 to 2016.
The age-standardized mortality rates of cervical cancer in females increased on average by 4.52% per year during from 2002 to 2016. The age-standardized mortality rates of pancreatic cancer, ovarian cancer, lymphoma, kidney cancer, and thyroid cancer were stable during from 2002 to 2016. The age-standardized mortality rates of colorectal cancer, gastric cancer, liver cancer, brain and other CNS tumors, esophageal cancer, and bladder cancer showed decreasing to varying degrees during from 2002 to 2016; notably, the average annual growth rate of esophageal cancer was –4.27% during the 2002–2009 period and –8.58% during the 2009–2016 period. The age-standardized mortality rates of breast cancer increased on average by 1.92% per year during from 2002 to 2009 but decreased on average by 2.01% per year from 2009 to 2016. The age-standardized mortality rates of gallbladder cancer were stable during the 2002–2011 period but decreased on average by 4.44% per year from 2011 to 2016.
Discussion
After analyzed the cancer incidence and mortality in Shanghai in 2016, The average life expectancy of the household-registered residents in Shanghai reached 83.18 years in 2016 (9), which is close to 85 years; therefore, the cumulative rates from 0 to 84 years were newly added to better assess the lifetime probability of developing and dying from malignant tumors among Shanghai residents. It was found that the probability of developing cancer was 41.39% and that the probability of dying from cancer was 21.89%.
The data quality of key indicators which from Shanghai tumor registry were similar when compared to 2015 (10). The values of most incidence indicators increased for all malignancies; however, the cumulative incidence rate was 24.56% for females aged 0 to74 years in 2016, compared to 24.66% in 2015. The values of most mortality indicators decreased; however, the TASR (age 35–64 years) was 84.54% for females in 2016, compared to 84.52% in 2015. Thus, the overall risk of developing a cancer rose, and the risk of death from cancer declined. The comparison between males and females showed that the TASR (age 35–64 years) and cumulative incidence rate (age 0–64 years) were numerically higher in females than in males, although the other indicators were higher in males than in females in 2015. In terms of age groups, a trend in which both age-specific incidence and mortality rates increased with age was evident; in particular, the age-specific incidence rate was higher in females than in males in the 20–49-year group, while the age-specific incidence rate for the 60-year-and-older group and the age-specific mortality rate for the 50-year-and-older group were both higher in males than in females. Notably, the central trends in both numbers and rates of cancer incidence and mortality varied across different age groups, with the number of cases being the highest in the 60–64-year group and the incidence being the highest in the 80–84-year group, which may explain the assertion that an increasing number of younger adults are becoming susceptible to malignant tumors.
Based on the latest estimates of incidence and mortality rates of 36 cancer types in 185 countries and regions worldwide in the IARC’s GLOBOCAN 2020 database (1,11), the age-standardized incidence rate of malignant tumors in Shanghai was 231.58/100,000 in 2016, which was higher than the global average (201.0/100,000) and the Chinese average (204.8/100,000) in 2020. It was higher than the average levels in the high, medium, and low Human Development Index (HDI) countries, but lower than the average level (295.3/100,000) in countries with a very high HDI. The age-standardized mortality rate was 90.01/100,000, which was lower than the world average (100.7/100,000) and the Chinese average (129.4/100,000); it was higher than the average levels in the high, medium, and low HDI countries; lower than the average (113.7/100,000) of the high HDI countries; and comparable to the average (98.7/100,000) of the very high HDI countries. Gender-based comparisons showed similar results. It has been suggested that the prevalence of malignancies can be considered an important marker of socioeconomic development. In countries and regions undergoing major developmental transitions, the cancer incidence mostly increases with increasing HDI; however, the mortality rate decreases in very high HDI countries due to improved survival (12,13). The temporal trend analysis in our current study showed the age-standardized incidence rates increased in both males and females in Shanghai between 2009 and 2016, while the age-standardized mortality rates decreased between 2002 and 2016. During the same period, the HDI in Shanghai increased from 0.734 in 2000 to 0.854 in 2017, second only to Beijing among all the province-level territories, which was already at a very high HDI level (14). In addition, the current trends in cancer incidence and mortality are likely to persist as HDI continues to rise.
Comparison of the latest estimates of malignancy incidence and mortality by sub-region in China completed by the National Cancer Center (15), the age-standardized incidence rates for each gender dimension in Shanghai in 2016 were higher than the averages for China as a whole and by sub-region in 2015, and the age-standardized mortality rates were lower than the averages for China as a whole and by sub-region. Although the age-standardized incidence rates were still lower in females than in males in China both as a whole and by sub-region, it has been higher in females than in males in Shanghai since 2015. Notably, the incidence rate nationwide became higher in females than in males in all or most of the age subgroups within the 15–54-year group, and Shanghai also reflected this trend. The age-specific cancer incidence and mortality in Shanghai were also generally consistent with those in China both as a whole and by sub-region, regardless of gender. In terms of tumor spectrum and in comparison, to China as a whole, gastric, liver, esophageal, and cervical cancer ranked lower in Shanghai in terms of both incidence and mortality as did the ASRs of incidence and mortality; in contrast, the prostate and thyroid cancers ranked higher in males, with higher ASRs. The differences in tumor spectrum and ASRs were relatively smaller when compared with the Eastern region.
In contrast to previous reports (10,16), this article specifically presents the top 5 cancers with the highest incidence or mortality by common age groups in Shanghai in 2016. In recent years, cancer patients being of younger age has drawn considerable research concern, and more efforts have been made in cancer prevention and early detection in AYA. Analysis of cancer incidence and mortality by common age groups may be more intuitive. First, older adults remain the most affected age group in terms of both incidence and mortality, and their tumor spectrum is essentially the same that of the whole population. The 60–79-year group had the highest proportion of incidence and mortality (48.85% and 48.67%, respectively) and thus is the top priority population in cancer prevention and treatment programs. The proportions of incidence and mortality in the age group of 80 years and older were 18.37% and 34.81%, respectively; since this age group is close to the average life expectancy, it is more important to improve the quality rather than it is to prolong life. Middle-aged people in the 40–59 years of age form the working core of society, and the proportions of incidence and mortality in this age group were 26.46% and 15.17%, respectively. Although thyroid cancer ranks first in incidence in the 40–59-year group, controversies exist concerning its extremely low mortality rate and its over-diagnosis (17). Thus, the prevention and treatment of thyroid cancer may be less valuable than the management of other more serious cancers, such as breast, colorectal, gastric, liver, and lung cancer, with the latter ranking second in incidence and the first in mortality. These cancer types have early detection methods that have been validated or are in the process of validation (18). More awareness-raising activities should be carried out on these cancers, thus if conditions permit, early diagnosis techniques should be applied in employee health check-ups. While the proportions of incidence and mortality were low in children in the 0–14-year group and AYA in the 15–39-year group, thyroid cancer accounted for 52.54% of the cases. Awareness-raising activities should be carried out in preschool, compulsory and vocational settings, and these data may inform adolescent and child cancer treatment and research.
In addition to the overall cancer trends, we also analyzed the trends in the age-standardized incidence and mortality of the 16 most common cancers in Shanghai by gender between 2002 and 2016, which comprehensively reflected the changes in cancer risk factors, screening technology, diagnosis and treatment capacity in the Shanghai. For instance, Shanghai launched a citywide community-based cancer screening program in 2013 (19) although evidence for its mortality-reducing effect has been insufficient.
Cancers have diverse etiologies and can be affected by a variety of factors from oncogenesis to patient death. Thus, the results of this study only offer evidence for future clinical studies. As the incidence and death data in the Shanghai cancer registry that covers the entire Shanghai population have been accumulated for 15 years, we will continue to carry our more tailored investigations on the main cancer types and key age groups through further data mining with an attempt to contribute to the reduction of the cancer burden.
Due to the limited availability of data in China, comparisons with neighboring regions and similar urban areas in China were not conducted in this study.
Acknowledgments
We thank the staff of the disease prevention and control centers, tumor case reporting hospitals, and community health service centers in all districts of Shanghai for their hard work in data collection and quality assurance in the cancer registry.
This article is a translation based on an original article first reported in Chinese in China Oncology [2022;32(6):519-526], with permission acquired.
Funding: This article was supported by Shanghai Leading Medical Talents Program (No. 2019LJ24), “Big Data and Artificial Intelligence Application” Project under the Three-year Action Plan for the Construction of Shanghai Public Health System (No. GWV-10.1-XK05), the Shanghai Science and Technology Achievement Transformation and Industrialization Project, and Multi-omics Biological Profiling and Colorectal Cancer Risk Assessment in General Population and Colorectal Cancer Screening Population in Shanghai (No. 18401933403).
Footnote
Data Sharing Statement: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-40/dss
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-40/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This article did not require ethical approval, because it’s a study on surveillance system of notifiable diseases by law.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Cancer Center. Guideline for Chinese Cancer Registration, 2016 edition. Beijing: People's Medical Publishing House, 2016:59-75.
- Parkin DM, Chen VW, Ferlay J, et al. Comparability and quality control in cancer registration. IARC technical report No 19. Lyon: IARC Press, 1994.
- Shanghai Center for Disease Control and Prevention. Incidence, mortality and survival of malignant tumors in Shanghai: 2001-2012. Shanghai: Shanghai Science Popularization Press, 2017.
- Segi M. Cancer mortality for selected sites in 24 countries (1950-1957). Sendai, Japan: Tohoku University School of Medicine, 1960.
- Jensen OM, Parkin DM, Maclennan R, et al. Cancer registration: principles and methods. Lyon IARC Scientific Publications 1991;
- Joinpoint Regression Program, Version 4.8.0.1[CP]. Statistical Research and Applications Branch, National Cancer Institute. April, 2020.
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Zheng Y, Wang CF, Wu CX, et al. Interpretation of three main indicators of the “2030 Plan for Healthy Shanghai”. Shanghai Journal of Preventive Medicine 2018;30:11-4.
- Bao PP, Wu CX, Zhang ML, et al. Report of cancer epidemiology in Shanghai, 2015. China Oncology 2019;29:81-99.
- Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: Cancer today[DB/OL]. Lyon, France: International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today (accessed 2021-07-15).
- Bray F. Transitions in human development and the global cancer burden. In: Stewart BW, Wild CP, editors. World cancer report 2014. Lyon: IARC Press, 2014:42-55.
- Fidler MM, Soerjomataram I, Bray F. A global view on cancer incidence and national levels of the human development index. Int J Cancer 2016;139:2436-46. [Crossref] [PubMed]
- UNDP, China Institute for Development Planning of Tsinghua University, State Information Centre joint task force. In pursuit of a more sustainable future for all: China's historic transformation over four decades of human development. Beijing: China Translation Press, 2019.
- Sun KX, Zheng RS, Zhang SW, et al. Report of cancer incidence and mortality in different areas of China, 2015. China Cancer 2019;28:1-11. [PubMed]
- Bao PP, Gong YM, Peng P, et al. Analysis of cancer incidence and mortality in Shanghai, 2014. China Oncology 2018;28:161-76.
- Li M, Dal Maso L, Vaccarella S. Global trends in thyroid cancer incidence and the impact of overdiagnosis. Lancet Diabetes Endocrinol 2020;8:468-70. [Crossref] [PubMed]
- Wender RC, Brawley OW, Fedewa SA, et al. A blueprint for cancer screening and early detection: Advancing screening's contribution to cancer control. CA Cancer J Clin 2019;69:50-79. [Crossref] [PubMed]
- Gong Y, Peng P, Bao P, et al. The Implementation and First‐Round Results of a Community‐Based Colorectal Cancer Screening Program in Shanghai China. The Oncologist 2018;23:928-35. [Crossref] [PubMed]
Cite this article as: Wu C, Gu K, Pang Y, Bao P, Wang C, Shi L, Gong Y, Xiang Y, Dou J, Wu M, Shi Y, Fu C. Cancer incidence and mortality in Shanghai: rates in 2016 and trends from 2002 to 2016. Precis Cancer Med 2022;5:35.