
Outcomes of head and neck angiosarcoma with different treatment modalities: a 20-year single institutional experience
Introduction
Angiosarcoma (AS) is a rare cancer of the vascular endothelium, constituting 1.6–5.0% of all cutaneous soft tissue sarcomas. It commonly occurs in the head and neck region (1,2). The disease usually presents innocuously as a benign bruise-like lesion, often covered by the hairline (2,3), making diagnosis challenging. Surgically, it is difficult to achieve negative margins due to multifocality and subclinical infiltration (2,4). The disease often metastasizes to regional nodes and distant sites (lung, liver and bone) (1,4). As a result, the outcomes for head and neck AS are often poor, with 5-year survival ranging from 10% to 54% (2).
Due to its rarity (4), there is a paucity of related literature. As most studies were presented as case reports and series, there is a lack of established management consensus. While some studies advocated surgery with adjuvant radiotherapy (2,5-8), many other studies showed otherwise, particularly for larger tumors (1,4,6,7,9). For example, a study interrogated the SEER database and concluded that surgery and radiotherapy were not associated with survival (10). A few authors also demonstrated extended survival in selected patients, who only received chemotherapy with or without local radiotherapy (2,5-7).
Other centers had reported different treatment strategies as well. A European study examined the possibility of neoadjuvant chemotherapy for non-metastatic patients with AS (8). Various experimental techniques like photodynamic therapy (PDT) had been utilized for skin relapses too (11). A few recent publications also advocated for personalized medicine through molecular profiling (9,12). Researchers from the Angiosarcoma Project found UV-related mutational signatures in AS. These signatures were similar in other skin cancers, such as melanoma, and might explain the immunotherapy sensitivity of AS (13).
However, most of these studies were in Western context. These studies generally had patients with smaller and more operable tumors (2,11,12). For instance, a study in Mayo clinic reported that 73% of patients (n=40/55) with localized AS had multimodality therapies including surgery, with a 5-year locoregional control and overall survival (OS) of 18% and 38% respectively (2). In contrast, a few Asian series reported relatively lower survival outcomes (14-16). Given the dismal survival outcomes of Asian patients, it is essential to examine this disease in the Asian setting.
Hence, we aim to contribute to the literature our experience on head and neck AS, within a single Asian tertiary center. We will report outcomes from various treatment modalities and explore the prognostic significance of blood inflammatory markers. We present the following article in accordance with the STROBE reporting checking (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-40/rc).
Methods
This was a retrospective cohort study from a larger prospectively collected sarcoma database. Patients with scalp and face AS treated in National Cancer Centre Singapore were included. The year of diagnosis ranged from 1999 to 2020. The study was approved by institutional review board (Singhealth IRB 2018/3065, 2018/2020), with waiver of consent for patients lost to follow up, and written informed consent obtained from the patients whenever possible. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Tumor size was categorized at 5-cm cut-off, in accordance with the American Joint Committee on Cancer (AJCC) TNM criteria. Patients were classified as metastatic if extensive multifocal lesions or distant metastases were presented at diagnosis. Blood biomarkers (hemoglobin, platelets, and white cell count) were collected at time of diagnosis to generate inflammatory ratios. Relapses were diagnosed based on computed tomography (CT) scan or clinical assessment of lesion size and numbers. Death-related data was obtained from the National Registry.
Statistical analysis
Survival outcomes examined for the study were OS and progression free survival (PFS). To determine significant factors for survival outcomes, univariable and multivariable cox proportional hazard model analyses were performed. The following variables were examined as potential factors for survival outcomes: age at diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status, tumor size, metastatic at diagnosis, sex, surgery and blood biomarkers. OS was defined as the time of diagnosis to time of last follow up/death. PFS was defined as the time of diagnosis to time of first recurrence/last follow up. Outcomes were also described for three sub-groups of patients: (I) patients who underwent surgery with a curative intent; (II) patients with localized tumours without radical resection; (III) patients with de novo metastases.
All statistical analyses were done using R (Version 4.0.3), assuming the tests were two-sided and at 5% significance level.
Results
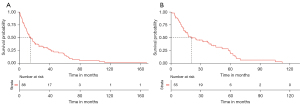
A total of 88 patients were included in the study. Table 1 summarized the patients’ characteristics. The mean age was 73.7 years [Standard deviation (SD): 12.2]. Most patients were male (n=62), ethnic Chinese (n=78), and with ECOG 0–1 (n=67). Thirty-one patients were metastatic at diagnosis. Fifty-five patients had localized disease at diagnosis and two patients did not have staging assessment due to frailty. Of patients without distant metastases, 22 patients had tumor smaller than 5 cm; 33 patients had large or extensive disease. Most patients (n=74) had primary tumor arising from the scalp. The median follow-up was 12.8 months [interquartile range (IQR): 5.7–25.9 months]. The 2- and 5-year OS were 37.5% (95% CI: 28.4–49.5%) and 14.7% (95% CI: 8.13–26.6%) respectively. The median OS was 14.7 months (95% CI: 10.4–21.2 months) (Figure 1A).
Table 1
| Variable | n (%) |
|---|---|
| Mean age at diagnosis (SD) (n=88) | 73.7 (12.2) |
| Sex (n=88) | |
| Female | 26 (29.5) |
| Male | 62 (70.5) |
| Ethnicity (n=88) | |
| Chinese | 78 (88.6) |
| Malay | 6 (6.8) |
| Indian | 1 (1.1) |
| Others | 3 (3.3) |
| ECOG Performance Status at diagnosis (n=82) | |
| 0 | 34 (41.5) |
| 1 | 33 (40.2) |
| 2 | 6 (7.3) |
| 3 | 5 (6.1) |
| 4 | 4 (4.9) |
| Mets at diagnosis (n=86) | |
| No | 55 (64.0) |
| Yes | 31 (36.0) |
| Size of tumor (n=86) | |
| <5 cm | 22 (25.6) |
| ≥5 cm | 33 (38.4) |
| Metastatic | 31 (36.0) |
| Site of tumor (n=88) | |
| Scalp | 74 (84.1) |
| Face and neck | 14 (15.9) |
| Grade (n=41) | |
| 2 | 20 (48.8) |
| 3 | 21 (51.2) |
| Median OS in months (95% CI) | 14.7 (10.4–21.2) |
n represents the sample size. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; SD, standard deviation.
Types of treatment
Surgery
Twenty-one patients underwent surgery with curative intent: seven patients with R1, the rest R0. One patient had initial nodal dissection. Another patient had nodal dissection upon relapse. At time of study, all patients had relapsed, except for two patients who were disease free. For these two patients, they had small tumors that were excised completely without adjuvant treatment. For first site of relapse, 15 cases were locoregional. Additionally, one case had local and distant relapse simultaneously; three cases had distant relapse.
Radiotherapy
Seven patients had adjuvant radiotherapy: five cases after R0 and two cases after R1. Fifty-one patients had palliative radiotherapy, either sequentially after chemotherapy, or by itself. Of assessable patients, none had lesions that progressed during treatment; all had documented response or stable disease. The median RT dose given for adjuvant RT was 56 Gy (IQR: 55–60 Gy) and the median RT fraction was 29 (IQR: 22–30). The palliative regimen ranged from single hypo-fractionated (e.g., single 8 Gy) to high dose conventional treatments (e.g., 60 Gy in 30 fractions).
Chemotherapy
None received (neo)adjuvant chemotherapy. Chemotherapy was administered for unresectable disease, or upon relapse (n=64). Most patients were given taxanes (n=36). Subsequently, 18 patients were given 2nd line chemotherapy, 13 patients were given 3rd-line chemotherapy and three patients were given 4th line chemotherapy. Of 35 assessable patients after first line chemotherapy, six patients had documented progression of lesions, 23 patients had good response, six patients had stable lesions.
PDT
Six patients had PDT for cutaneous relapses. Five patients had locoregional or distant progressive disease. One had abscopal effect with partial response of distant disease after primary site PDT.
Outcomes of patients with localized disease
Table 2 summarized the patients’ characteristics with localized (non-metastatic) disease at presentation (n=55). Thirty-seven patients passed away from AS. The 2-year and 5-year OS were 49.9% (95% CI: 37.9–65.7%) and 18.9% (95% CI: 9.6–37.1%) respectively. The median OS was 20.4 months (95% CI: 14.8–45.1 months) (Figure 1B). The 2-year and 5-year PFS were 51.2% (95% CI: 36.6–71.6%) and 20.4% (95% CI: 8.5–48.7%) respectively. The median PFS was 26.3 months (95% CI: 19.2–37.4 months).
Table 2
| Variable | n (%) |
|---|---|
| Mean age at diagnosis (SD) (n=55) | 72.9 (12.2) |
| ECOG Performance Status at diagnosis (n=51) | |
| 0 | 27 (52.9) |
| 1 | 17 (33.3) |
| 2 | 4 (7.8) |
| 3 | 1 (2.0) |
| 4 | 2 (3.9) |
| Size of tumor (n=55) | |
| <5 cm | 22 (40.0) |
| ≥5 cm | 33 (60.0) |
| Site of tumor (n=55) | |
| Scalp | 46 (83.6) |
| Face & neck | 9 (16.4) |
| Death (n=55) | |
| No | 13 (23.6) |
| Yes | 42 (76.4) |
| Death due to angiosarcoma (n=42) | |
| No | 5 (11.9) |
| Yes | 37 (88.1) |
| Relapse (Locoregional or Distant) (n=55) | |
| No | 30 (54.5) |
| Yes | 25 (45.5) |
| Median OS in months (95% CI) | 20.4 (14.8–45.1) |
| Median PFS in months (95% CI) | 26.3 (19.2–37.4) |
| Patients with surgery (Curative intent) (n=21) | |
| Surgical margins (n=21) | |
| R0 | 14 (66.7) |
| R1 | 7 (33.3) |
| Adjuvant radiotherapy (n=21) | |
| Yes | 7 (33.3) |
| No | 14 (66.7) |
n represents the sample size. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; SD, standard deviation.
Twenty-one patients had surgery with curative intent. Of these patients, 14 patient had negative margins and seven patients had adjuvant radiotherapy. More local relapses were observed for patients with adjuvant radiotherapy (57.1%), as compared to those without adjuvant radiotherapy (35.7%). Patients with adjuvant radiotherapy had better OS as compared to those without adjuvant radiotherapy (log-rank test P=0.002).
When the patients were stratified by surgery, patients who had surgery with curative intent were younger [mean age: 70.1 (surgery) vs. 75.1 (no surgery), P=0.142], with better ECOG [ECOG 0: 84.2% (surgery) vs. 35.5% (no surgery), P<0.01] and smaller tumour size [tumour size <5 cm: 71.4% (surgery) vs. 21.2% (no surgery), P<0.01].
Outcomes of patients with de novo metastases
For patients with metastatic disease at presentation (n=31, Table 3), the mean age is 73.6 (SD: 10.8) years. Most of the patients were ECOG 1 (n=16). All but one patient died from AS. The median follow-up was 6.9 (IQR: 2.6–19.2) months. The median OS was 6.9 (95% CI: 3.7–19.0) months. In terms of treatments given, 17 patients had palliative radiotherapy and 17 patients had palliative chemotherapy. Among the patients who had palliative chemotherapy, most demonstrated response. Among patients who were given palliative RT and assessable (n=8), all had response to the treatment.
Table 3
| Variable | n (%) |
|---|---|
| Mean age at diagnosis (SD) (n=31) | 73.6 (10.8) |
| ECOG Performance Status at diagnosis (n=29) | |
| 0 | 7 (24.1) |
| 1 | 16 (55.2) |
| 2 | 2 (6.9) |
| 3 | 3 (10.3) |
| 4 | 1 (3.4) |
| Site of tumor (n=31) | |
| Scalp | 27 (87.1) |
| Face & neck | 4 (12.9) |
| Grade (n=12) | |
| 2 | 2 (16.7) |
| 3 | 10 (83.3) |
| Death due to angiosarcoma (n=31) | |
| No | 1 (3.2) |
| Yes | 30 (96.8) |
| Median OS in months (95% CI) | 6.9 (3.7–19.0) |
| Palliative Radiotherapy (n=31) | |
| No | 14 (45.2) |
| Yes | 17 (54.8) |
| Palliative Chemotherapy (n=31) | |
| No | 14 (45.2) |
| Yes | 17 (54.8) |
n represents the sample size. CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; OS, overall survival; SD, standard deviation.
Selected non-surgical patients with extended survival
Among patients who did not have surgery with a curative intent, 14 patients had OS of more than two years. The median age was 72. Four patients were metastatic at diagnosis, six patients had large tumours, three patients had small tumours of <5 cm. For treatment, six patients received chemoradiotherapy, two patients received chemoradiotherapy with PDT, one patient received chemotherapy with PDT, three patients received chemotherapy alone, two patients only had palliative radiotherapy. At initial biopsy, seven patients had well differentiated AS, seven patients were grade 2 and two patients were grade 3. Seven patients did not have their tumour grade assessed. For survival outcomes, 11 patients passed away from AS, one patient passed away from subdural hemorrhage, and two patients were alive at time of analysis. Amongst dead patients (n=12), the median OS was 47.0 months.
OS and PFS for the different groups of patients
For overall survival of patients with (I) de novo metastatic AS; (II) curative surgery for localized disease; (III) no curative surgery for localized disease (Figure 2A), there was a significant difference in OS among the three groups (P<0.001). Patients with curative surgery for localized disease had the highest OS, while patients with metastatic AS had lowest OS. For patients with curative surgery for localized disease, the 2- and 5-year OS were 64.6% (95% CI: 46.6–89.6%) and 33.2% (95% CI: 15.7–70.3%) respectively. Pertaining to patients with no curative surgery for localized disease, the 2-year and 5-year OS were 42.0% (95% CI: 27.6–63.9%) and 10.0% (95% CI: 2.81–35.6%) respectively.

Of patients with localized tumours, the 2-year PFS of patients with no curative surgery was significantly higher than those with curative surgery (62.8% vs. 35.0%, P=0.026) (Figure 2B).
Cox proportional hazard model for OS and PFS
Univariable analysis of OS for all patients (Table 4) revealed that age [hazard ratio (HR): 1.04; 95% CI: 1.02–1.07], ECOG (HR of ECOG 1 vs. ECOG 0: 2.08; 95% CI: 1.22–3.57, HR of ECOG 2–4 vs. ECOG 0: 3.84; 95% CI: 1.99–7.43), tumor size (HR of metastatic vs. <5 cm: 2.50; 95% CI: 1.40–4.53), metastasis at diagnosis (HR of metastasis vs. no metastasis: 1.90; 95% CI: 1.18–3.06), grade of tumour (HR of grade 3 vs. grade 2: 2.24; 95% CI: 1.10–4.56) and surgery (HR of surgery vs. no surgery: 0.49; 95% CI: 0.28–0.88) were significant predictors for OS (Table 4). When these predictors were included in the multivariable model (Table 4), only age (HR: 1.04; 95% CI: 1.01–1.07) and metastasis at diagnosis (HR: 1.98; 95% CI: 1.09–3.61) were shown to be significant (Table 4).
Table 4
| Variable | Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at diagnosis (n=88) | 1.04 (1.02–1.07) | <0.001 | 1.04 (1.01–1.07) | 0.015 | |
| ECOG Performance Status (Reference =0) (n=82) | |||||
| 1 | 2.08 (1.22–3.57) | <0.001 | 1.68 (0.91–3.08) | 0.097 | |
| 2–4 | 3.84 (1.99–7.43) | <0.001 | 2.31 (0.98–5.42) | 0.055 | |
| Tumor size (Ref: <5 cm) (n=86) | |||||
| ≥5 cm | 1.74 (0.93–3.24) | 0.082 | |||
| Metastatic | 2.50 (1.40–4.53) | 0.003 | |||
| Metastasis at diagnosis (Reference: no) (n=86) | |||||
| Yes | 1.90 (1.18–3.06) | <0.001 | 1.98 (1.09–3.61) | 0.025 | |
| Margin (Reference: negative) (n=20) | |||||
| Positive | 3.00 (1.00–9.04) | 0.051 | |||
| Sex (Reference: female) (n=88) | |||||
| Male | 0.89 (0.54–1.45) | 0.632 | |||
| Grade (Reference: Grade 2) (n=41) | |||||
| Grade 3 | 2.24 (1.10–4.56) | 0.026 | |||
| Surgery (Reference: No) (n=84) | |||||
| Yes | 0.49 (0.28–0.88) | 0.017 | 1.09 (0.52–2.28) | 0.825 | |
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Univariable analysis of PFS for patient with localized AS showed that only surgery was a significant predictor for PFS (HR of surgery vs. no surgery: 2.30; 95% CI: 1.15–4.60).
Cox proportional hazard model for OS with regards to blood biomarkers
Lower hemoglobin level (HR: 0.79; 95% CI: 0.68–0.92) and lower lymphocyte:monocyte ratio (LMR) (HR: 0.81; 95% CI: 0.67–0.97) were significantly associated with worse survival (Table 5). When hemoglobin, LMR, age and metastatic status were included in the multivariable analysis of OS (Table 5), the blood biomarkers no longer predicted for survival.
Table 5
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Hemoglobin (n=67) | 0.79 (0.68–0.92) | 0.003 | 0.89 (0.74–1.08) | 0.237 | ||
| NLR (n=72) | 1.02 (1.00–1.05) | 0.062 | ||||
| PLR (n=72) | 1.001 (0.999–1.002) | 0.568 | ||||
| LMR (n=72) | 0.81 (0.67–0.97) | 0.025 | 0.84 (0.70–1.01) | 0.070 | ||
| Age at diagnosis (n=65) | 1.04 (1.00–1.08) | 0.028 | ||||
| Metastasis at diagnosis (Ref: No) (n=65) | ||||||
| Yes | 1.66 (0.93–2.95) | 0.088 | ||||
CI, confidence interval; HR, hazard ratio; NLR, neutrophil-lymphocyte ratio; PLR, platelet lymphocyte ratio; LMR, lymphocyte-monocyte ratio.
Discussion
Our study presented the following key findings: (I) 2- and 5-year OS of patients with head and neck AS were guarded 37.5% and 14.7%; (II) patients who underwent surgery with a curative had worse PFS but better OS; (III) most surgical patients experienced locoregional recurrences as first site of relapse; (IV) some non-surgical patients were able to survive beyond two years; (V) there were good responses on taxanes; (VI) only age and clinical stage were significant predictors of OS.
Our study demonstrated that AS is aggressive, as its 2-year (49.9%) and 5-year OS (18.9%) of localized disease were dismal. These findings were in line with some of the existing literature (1,15,16). However, our study had poorer outcomes as compared to Western reports. A study from Mayo clinic showed that the 5-years for patients with localized AS was 41% (2). Another study from University of Florida revealed that the 5-year OS of patients with AS was 54% (17).
From our study, patients who had surgery with curative intent had better OS but worse PFS. After adjusting for age, performance status and metastatic status at presentation, association of OS with surgery was not significant. The finding of worse PFS could be explained by closer surveillance and hence earlier detection of recurrences. Non-surgical patients were also on palliative chemotherapy, which could have delayed relapses (none of our surgical patients received (neo)adjuvant chemotherapy). Some studies had similar findings, postulating that post-surgical inflammatory marks like vascular endothelial growth factor (VEGF) can cause tumours proliferation (18). Fifteen of 21 surgical patients experienced locoregional recurrences as first site of relapse. These findings support the emerging perspective that morbid surgery should be avoided due to high recurrence rate (2,8). Other studies have corroborated that risks of distant metastases and locoregional relapses after surgery were high (19). Moreover, a Canadian series could not find a difference in outcomes between surgery alone, radiotherapy alone or combined treatment (1).
In light of high relapse rates, some authors have proposed simple resection followed by adjuvant radiotherapy (2,20). However, our study implied otherwise. In our results, there was a higher proportion of relapses for surgical patients with adjuvant radiotherapy [57.1% (adjuvant radiotherapy) vs. 35.7% (without adjuvant radiotherapy)]. Hence, it remains debatable whether resection and adjuvant radiotherapy should be routinely offered in these patients. Such an approach can be morbid and logistically challenging, especially in elderly or frail patients. However, in smaller localized tumours, wide excision may still have a role if it can be achieved without overt complications (2,20).
Our study showed that among non-surgical patients, 14 patients managed to achieve long-term survival when treated conservatively. Other published studies also support our finding, showing that for treatment-sensitive tumours, chemotherapy and radiation effects can be long-lasting (2,5,6). Outcomes from Asian researchers have also found that many lesions were too extensive and more likely to have subclinical distant/locoregional disease. For these cases, chemotherapy and/or radiotherapy may be more suitable. For example, Fujisawa et al. compared patients treated with surgery vs. taxane-based conservative therapy and found that the latter had better outcomes (21). In another study by Fujisawa et al. (7), patients who had surgery had a lower 5-year OS (8%) as compared to those with chemo-radiotherapy (56%). On the contrary, a small series from Tokyo that employed high dose radiotherapy with taxane reported median survival of only 20 months, with patients succumbing to locoregional and pulmonary relapses (22). Hence, the future challenge is in profiling and predicting good responders to better customize therapy.
In the unresectable or relapsed setting, we reported good response to taxanes. The use of taxanes was also recommended in a review article by Erikssen (23). Paclitaxel is a better tolerated agent compared to doxorubicin, especially in elderly patients who may have existing cardiac condition (23). Radiation has been reported to be ineffective as a curative monotherapy and is generally used for palliation (20). In our study, there were no instances of radiation-resistant AS. We recommend palliative chemotherapy, with or without radiotherapy for consolidation. However, we did not report on the duration of chemotherapy and did not study the utility of maintenance/extended chemotherapy, although described by small series to be useful (6).
In our study, multivariable analysis revealed that only age and stage were significant predictors for OS, similar to other studies (2). For the various blood biomarkers, hemoglobin and lymphocyte-monocyte ratio were significant prognostic factors in univariable analysis. However, they were shown not to be statistically significant in the multivariable analysis. This lack of statistical significance might be attributed to our smaller sample size.
While blood biomarkers were not significant factors in our study, recent studies had examined the molecular profiles of AS. In a larger study led by one of our co-authors, subtypes of AS are observed based on gene expressions in inflammation-related pathways (9). In another study by Chan et al., it examined a large cohort of AS from which patients in this study were a subset of. He found that non-responders to chemotherapy had higher oncogenic pathway scores. Moreover, he found that high peripheral blood NLR was correlated with intra-tumoral NLR and was associated with worse outcomes (24). These findings suggest that treatments that target oncogenic pathways, such as immunotherapy, may be helpful in treating the disease. Interestingly, one of our patients had documented abscopal response after PDT, further highlighting possible immunogenicity with AS (11).
Our study has several strengths. Firstly, it contributes significantly to the limited number of smaller Asian studies. Secondly, death data was corroborated from the National Registry. Thirdly, we reported on outcomes with different modalities in both localized and metastatic patients. Lastly, it is one of the few studies that examined the characteristics and treatment responses of metastatic patients.
Limitations include the retrospective nature of our study and limited generalizability as the findings are from a single institution. Thirdly, though multivariable analyses were attempted to account for potential confounders, the statistical power was limited by small sample size. Fourthly, it was difficult to objectively assess tumour response to various therapies as lesions were cutaneous and can be subtle, and often not obvious on imaging. Especially when assessing response after palliative radiotherapy or PDT, it could be difficult to tell dermatitis apart from disease. Lastly, we did not analyze sequencing of chemotherapy and local treatment, and cycles of chemotherapy (maintenance chemotherapy). Based on experience, most patients would receive chemotherapy first to prevent severe toxicities from concurrent multimodality treatments.
Conclusions
We showed that head and neck AS had poor outcomes. There was a high risk of locoregional relapses and AS-deaths, even in patients with localized disease and after curative surgery. Age and stage were shown to be significant factors for overall survival. Although we found that surgery was associated with better OS, it could have been confounded by other factors. The utility of adjuvant radiotherapy remains debatable as it is difficult to draw conclusions from uncontrolled retrospective series. For smaller tumours, while wide excision with or without adjuvant radiotherapy is an option, the clinical benefit of which compared to a more conservative approach with upfront chemotherapy remains unanswered. Selected patients with unresectable disease may have long lasting response from chemotherapy, with or without radiotherapy. The future challenge lies in profiling and predicting for treatment-sensitive tumours, and to determine the duration and sequencing of chemotherapy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-40/rc
Data Sharing Statement: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-40/dss
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-40/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by institutional review board (Singhealth IRB 2018/3065, 2018/2020), with waiver of consent for patients lost to follow up, and written informed consent obtained from the patients whenever possible. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bernstein JM, Irish JC, Brown DH, et al. Survival outcomes for cutaneous angiosarcoma of the scalp versus face. Head Neck 2017;39:1205-11. [Crossref] [PubMed]
- Patel SH, Hayden RE, Hinni ML, et al. Angiosarcoma of the scalp and face: the Mayo Clinic experience. JAMA Otolaryngol Head Neck Surg 2015;141:335-340. [Crossref] [PubMed]
- Köhler HF, Neves RI, Brechtbühl ER, et al. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg 2008;139:519-24. [Crossref] [PubMed]
- Lee KC, Chuang SK, Philipone EM, et al. Characteristics and Prognosis of Primary Head and Neck Angiosarcomas: A Surveillance, Epidemiology, and End Results Program (SEER) Analysis of 1250 Cases. Head Neck Pathol 2019;13:378-85. [Crossref] [PubMed]
- Miki Y, Tada T, Kamo R, et al. Single institutional experience of the treatment of angiosarcoma of the face and scalp. Br J Radiol 2013;86:20130439. [Crossref] [PubMed]
- Fujisawa Y, Yoshino K, Fujimura T, et al. Cutaneous Angiosarcoma: The Possibility of New Treatment Options Especially for Patients with Large Primary Tumor. Front Oncol 2018;8:46. [Crossref] [PubMed]
- Fujisawa Y, Yoshino K, Kadono T, et al. Chemoradiotherapy with taxane is superior to conventional surgery and radiotherapy in the management of cutaneous angiosarcoma: a multicentre, retrospective study. Br J Dermatol 2014;171:1493-500. [Crossref] [PubMed]
- Constantinidou A, Sauve N, Stacchiotti S, et al. Evaluation of the use and efficacy of (neo)adjuvant chemotherapy in angiosarcoma: a multicentre study. ESMO Open. 2020;5:e000787. [Crossref] [PubMed]
- Chan JY, Lim JQ, Yeong J, et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J Clin Invest 2020;130:5833-46. [Crossref] [PubMed]
- Conic RRZ, Damiani G, Frigerio A, et al. Incidence and outcomes of cutaneous angiosarcoma: A SEER population-based study. J Am Acad Dermatol 2020;83:809-16. [Crossref] [PubMed]
- Thong PS, Ong KW, Goh NS, et al. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol 2007;8:950-2. [Crossref] [PubMed]
- Painter CA, Jain E, Tomson BN, et al. The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med 2020;26:181-7. [Crossref] [PubMed]
- Boichard A, Wagner MJ, Kurzrock R. Angiosarcoma heterogeneity and potential therapeutic vulnerability to immune checkpoint blockade: insights from genomic sequencing. Genome Med 2020;12:61. [Crossref] [PubMed]
- Chow TL, Kwan WW, Kwan CK. Treatment of cutaneous angiosarcoma of the scalp and face in Chinese patients: local experience at a regional hospital in Hong Kong. Hong Kong Med J 2018;24:25-31. [Crossref] [PubMed]
- Chen TW, Pang A, Puhaindran ME, et al. The treatment landscape of advanced angiosarcoma in Asia-A multi-national collaboration from the Asian Sarcoma Consortium. Cancer Sci 2021;112:1095-104. [Crossref] [PubMed]
- Chan YH, Hsieh FN, Lin LY, et al. Cutaneous angiosarcoma–A report of 20 Taiwanese patients. Dermatologica Sinica 2018;36:143-5. [Crossref]
- Scott MT, Portnow LH, Morris CG, et al. Radiation therapy for angiosarcoma: the 35-year University of Florida experience. Am J Clin Oncol 2013;36:174-80. [Crossref] [PubMed]
- Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol 2008;97:74-81. [Crossref] [PubMed]
- Chang C, Wu SP, Hu K, et al. Patterns of Care and Survival of Cutaneous Angiosarcoma of the Head and Neck. Otolaryngol Head Neck Surg 2020;162:881-7. [Crossref] [PubMed]
- Guadagnolo BA, Zagars GK, Araujo D, et al. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 2011;33:661-7. [Crossref] [PubMed]
- Fujisawa Y, Nakamura Y, Kawachi Y, et al. Comparison between taxane-based chemotherapy with conventional surgery-based therapy for cutaneous angiosarcoma: a single-center experience. J Dermatolog Treat 2014;25:419-23. [Crossref] [PubMed]
- Katano A, Yamashita H, Nakagawa K. Radical radiotherapy for localized cutaneous angiosarcoma of the scalp. Mol Clin Oncol 2021;15:195. [Crossref] [PubMed]
- Eriksson M. Histology-driven chemotherapy of soft-tissue sarcoma. Ann Oncol 2010;21:vii270-6. [Crossref] [PubMed]
- Chan JY, Tan GF, Yeong J, et al. Clinical implications of systemic and local immune responses in human angiosarcoma. NPJ Precis Oncol 2021;5:11. [Crossref] [PubMed]
Cite this article as: Koh YS, Chan JY, Looi WS, Farid M, Iyer NG, Tay CAG, Sommat K, Wong RX. Outcomes of head and neck angiosarcoma with different treatment modalities: a 20-year single institutional experience. Precis Cancer Med 2022;5:2.

