
Efficacy of pembrolizumab in lung adenocarcinoma harboring non-V600E BRAF mutation: a case report
Introduction
V-raf murine sarcoma oncogene homolog B1 (BRAF-1) belongs to serine/threonine kinase family, though included in RAS/MAPK pathway for cell adhesion and spreading through mitogen activated kinases and extracellular signal-regulated protein kinase phosphorylation (i.e., MEK1/2, ERK1/2) (1).
BRAF inducing mutations constitutively maintain downstream kinase activity and pathway thus promoting cell proliferation and survival, as an oncogenic driver (1).
In relation to biochemical behavior V600E substitution in exon 15 highly increase BRAF activity and occur in almost 50% of cases, instead of non-V600E ones (distributed in exon 11 and 15) which show variable degrees of direct activation on MEK1/2: these kinds of mutation elicit downstream pathway through the trans-activation of Raf-1 proto-oncogene, serine/threonine kinase (CRAF) (2).
While non-V600E BRAF point mutations are associated with current or former smoking habit, sex-female and never smoking status set up a frequent profile for V600E variant finding (3).
Nevertheless, BRAF role in non-small cell lung cancer (NSCLC) prognosis is unclear, some studies emphasize a shorter progression-free survival (PFS) to platinum-based chemotherapy in patients with V600E mutated tumors, compared to those with non-V600E mutations (2). However, tumors harboring V600E BRAF mutations have larger benefit from targeted therapy: Double therapy association with BRAF and MEK inhibitors represents a new therapy with clinically meaningful antitumor activity and a manageable safety profile in naive patients, achieving objective response rate (ORR) of 64% and median PFS of 9.7 months (4). In contrast, most non-V600E BRAF mutations do not respond to BRAF inhibition (5). Therefore, targeted therapies are currently only approved for V600E mutations.
A new preclinical framework has reclassified BRAF mutations, including V600 and non-V600, into three functional classes based on kinase activity and signaling mechanism. It remains to establish whether BRAF functional class influences clinicopathologic characteristics and clinical outcomes (6).
Due to the low frequency of BRAF-mutant NSCLCs, the immunological characteristics and the efficacy of immune checkpoint inhibitors (ICIs) have not been extensively studied (7).
A real-world study supports the hypothesis that ICIs may have an efficacy in BRAF-mutant NSCLC patients similar to that observed in the overall NSCLC population (8).
The present case report aims at describing features and outcomes of G466E BRAF-mutated NSCLC patient treated with pembrolizumab.
This case adds to the limited current published literature on NSCLC harboring non-V600E BRAF mutations and suggests that immunotherapy is a reasonable treatment option.
We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/rc).
Cases presentation
The patient is a 79 years old male who performed diagnostic investigations in December 2019 due to recurrent dorsal pain. Spine magnetic resonance imaging (MRI) showed multiple pathological secondary lesions on the spine. A subsequent full body computed tomography (CT) revealed the presence of a solid lung mass in the lower left lobe (LLL) larger than 6 cm and pleural effusion, and bone metastases.
In April 2020, he underwent to pleural biopsy and talc-procedure on pleural leaflets. The diagnosis was lung adenocarcinoma. Programmed death-ligand 1 (PD-L1) immunohistochemistry (IHC) expression was 60%, and molecular tests excluded the presence of driver mutations on EGFR, ALK and ROS-1 genes. Comprehensive molecular profiling by next generation sequencing (NGS) identified the G466E BRAF mutation. Other cancer-related alterations founded were NF1 (Y2285fs*5; Y2640fs*3), TP53 (D281Y). The tumor resulted microsatellite-stable and tumor mutational burden (TMB) was 5.04 mutations-per-megabase.
A positron emission tomography (PET) CT scan performed in August 2020 confirmed IV stage disease: pleural, mediastinal nodal, adrenal and several bones metastases. Then, the patient started first line immunotherapy with pembrolizumab. Three months later, the PET CT scan showed impressive response of disease: metabolic and dimensional reductions of lung and pleural lesions, complete metabolic response of disease on mediastinal nodes, adrenal and bone metastases (Figure 1). Thus, the patient continued therapy with pembrolizumab.
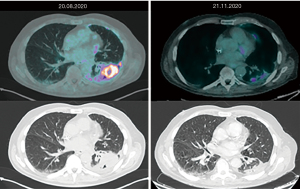
In February 2021, PET CT scan revealed a modest increase of the uptake on pleura, mediastinal lymph node and fourth-lumbar vertebra. However, in view of clinical benefit and radiological response achieved in all other sites of disease, he continued pembrolizumab treatment. The last PET CT performed 2 months later showed bone complete metabolic response and further partial metabolic response on lung, pleural and adrenal disease.
The patient is currently receiving treatment with pembrolizumab, maintaining clinical benefit and good safety profile.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Local ethics committee approval was not required due to non-experimental content of the manuscript.
Discussion
BRAF mutation is one of oncogenic driver mutations in NSCLC, which phosphorylates the downstream effectors MEK and ERK to promote cell proliferation and survival.
MAP Kinase pathway alteration is involved in several tumor types, thus allowing MEK inhibitors or pan-RAF inhibitors to target BRAF mutation in lung cancer. Moreover, MAPK-BRAF linked activation (including non-V600E variants) may be sensitive to specific signaling nodes of MEK and ERK (9).
Not yet provided a complete BRAF mutation list; only few papers referred other mutations in exon 11 and 15 (10).
According to classification system BRAF mutations are classified in three classes, G466E BRAF belonging to class III.
The clinical significance of class II–III mutation found in NSCLC remains unclear and may be associated with other upstream MAPK alterations, such as RAS mutation or neurofibromatosis gene (NF1) loss (11) or loss of function.
Changing of pathway by class III of alterations specifically maintain signals through dimerization with wild-type C-RAF. Upstream activation to increase ERK signaling is also requested, and obtained either through genomic alterations indeed (RAS mutations or NF1 loss) or through receptor tyrosine kinase (RTK) signaling. This upstream signaling will counter the ERK-mediated negative feedback on RAS proteins.
Class III BRAF mutations differentiate from class I and II ones for feedback of high RAS-GTP levels (12).
BRAF/MEK inhibition in non-V600E mutations mostly counts in melanoma patients according to clinical practice and case reports (13). Vemurafenib activity (a BRAF inhibitor) in patients with NSCLC harboring non-V600E and V600E BRAF mutations is slightly described in literature (14). High RAS levels present in cell-lines with this class of BRAF mutations should justify some more investigation on the combination of a MEK inhibitor plus a RTK inhibitor (such as EGFR) (15,16). Other way of investigation point towards ICIs still based on retrospective data and case reports. Rittberg et al. reported a case of a rare BRAF G469A mutated NSCLC successfully treated with nivolumab (17). Dudnik et al. retrospectively investigated the PD-L1 expression, the TMB, the microsatellite instability status, and the response to ICIs in BRAF-mutant NSCLC patients. BRAF mutations were associated with high level of PD-L1 expression, low/intermediate TMB and microsatellite-stable status. ICIs showed favorable activity in both BRAF V600E and BRAF non-V600E mutant NSCLC, with an ORR of 25% and 33%, respectively (18). Similarly, Guisier et al. suggest that the efficacy of ICIs in patients with actionable mutations and in unselected population is similar (19). The Authors emphasize that among 44 BRAF-mutated patients (of whom 26 were V600E mutated) the ORR was approximately 30% (33% for non-V600E, 26% for V600E mutated patients).
BRAF-mutations could more closely mirror the impact of KRAS mutations than EGFR or ALK alterations in NSCLC. Indeed, given BRAF’s association with smoking, PD-L1 expression and a higher mutational burden, there is a biological rationale for a higher sensitivity to ICIs compared to other oncogenic drivers (20).
We present an example of good tolerance and response to immunotherapy in a non-V600E mutated patient. This case shows once again the importance of driver mutations, in order to identify the most accurate therapeutic strategy, although not always possible due to lacking evidences. Indeed, due to increased sensitivity of NGS, more mutations of unknown significance are identified in clinical practice. Moreover, immunotherapy in NSCLC patients with driver mutations has quite low level of evidence of support yet, being rigorous judgment on benefit-harm balance still needed for clinical decision.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine, for the series “Uncommon Mutations in Non-Small Cell Lung Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/coif). The series “Uncommon Mutations in Non-Small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. MR served as the unpaid Guest Editor of the series. MR serves as an unpaid editorial board member of Precision Cancer Medicine from August 2020 to July 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Local ethics committee approval was not required due to non-experimental content of the manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen D, Zhang LQ, Huang JF, et al. BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e101354. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the European EURAF cohort. J Thorac Oncol 2015;10:1451-7. [Crossref] [PubMed]
- Lin Q, Zhang H, Ding H, et al. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J Transl Med 2019;17:298. [Crossref] [PubMed]
- Blons H, Garinet S, Laurent-Puig P, et al. Molecular markers and prediction of response to immunotherapy in non-small cell lung cancer, an update. J Thorac Dis 2019;11:S25-36. [Crossref] [PubMed]
- Rihawi K, Giannarelli D, Galetta D, et al. BRAF mutant NSCLC and immune checkpoint inhibitors: results from a real-world experience. J Thorac Oncol 2019;14:e57-9. [Crossref] [PubMed]
- Sheikine Y, Pavlick D, Klempner SJ, et al. BRAF in lung cancers: analysis of patient cases reveals recurrent BRAF mutations, fusions, kinase duplications, and concurrent alterations. JCO Precis Oncol 2018;2:PO.17.00172.
- Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016;91:23-8. [Crossref] [PubMed]
- Dagogo-Jack I, Martinez P, Yeap BY, et al. Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF-mutant lung cancer. Clin Cancer Res 2019;25:158-65. [Crossref] [PubMed]
- Frisone D, Friedlaender A, Malapelle U, et al. A BRAF new world. Crit Rev Oncol Hematol 2020;152:103008. [Crossref] [PubMed]
- Dankner M, Lajoie M, Moldoveanu D, et al. Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin Cancer Res 2018;24:6483-94. [Crossref] [PubMed]
- Mazieres J, Montané L, Barlesi F, et al. OA12. 05 Vemurafenib in patients harboring V600 and non V600 BRAF mutations: final results of the NSCLC cohort from the AcSé trial. J Thorac Oncol 2018;13:S348-9. [Crossref]
- Kotani H, Adachi Y, Kitai H, et al. Distinct dependencies on receptor tyrosine kinases in the regulation of MAPK signaling between BRAF V600E and non-V600E mutant lung cancers. Oncogene 2018;37:1775-87. [Crossref] [PubMed]
- Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234-8. [Crossref] [PubMed]
- Rittberg R, Banerji S, Green S, et al. Immunotherapy benefit in a patient with non-small cell lung cancer and a rare BRAF mutation. Cureus 2020;12:e11224. [Crossref] [PubMed]
- Dudnik E, Peled N, Nechushtan H, et al. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol 2018;13:1128-37. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and safety of anti–PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Addeo A, Passaro A, Malapelle U, et al. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev 2021;96:102179. [Crossref] [PubMed]
Cite this article as: Di Fazio GR, Citarella F, Dell’Aquila E, Russano M, Galletti A, Santo V, Vincenzi B, Tonini G, Santini D. Efficacy of pembrolizumab in lung adenocarcinoma harboring non-V600E BRAF mutation: a case report. Precis Cancer Med 2022;5:10.

