帕博利珠单抗治疗非V600E BRAF突变肺腺癌的疗效:病例报告
介绍
BRAF−1属于丝氨酸/苏氨酸激酶家族,是RAS/MAPK通路中的一员,通过丝裂原活化激酶和细胞外信号调节蛋白激酶磷酸化(即MEK1/2、ERK1/2)介导细胞粘附和扩散[1]。
BRAF突变是致癌驱动因子,可以维持下游激酶和通路的活性,从而促进细胞增殖和存活[1]。
就生物学行为而言,15号外显子中V600E碱基替换显著提高了BRAF活性,并且在50%的病例中出现。而11号或15号外显子上的非V600E突变则在不同程度上激活MEK1/2,这些突变通过反式激活Raf−1原癌基因CRAF来激活下游通路[2]。
非V600E BRAF点突变与现在或既往的吸烟习惯有关,而女性和无吸烟史人群与V600E突变相关[3]。
然而,BRAF在NSCLC预后中的作用尚不清楚。一些研究发现,与非V600E突变患者相比,V600E突变患者接受铂类化疗的无进展生存期(PFS)较短[2]。然而,携带V600E BRAF突变的肿瘤从靶向治疗中获益也更大。BRAF和MEK抑制剂的联合治疗代表了一种新的治疗方法,具有临床意义的抗肿瘤活性,并且在未成年患者中具有可控的安全性,达到了64%的客观反应率(ORR)和9.7个月的中位PFS[4]。相反,大多数非V600E BRAF突变对BRAF抑制没有反应[5]。因此,靶向治疗目前只被批准用于V600E突变。
一个新的临床前框架将BRAF突变(包括V600和非V600)根据激酶活性和信号传导机制重新划分为三个功能类别。BRAF功能类别是否会影响临床病理特征和临床结果仍有待确定[6]。
由于BRAF突变型的NSCLC的发生率较低,免疫检查点抑制剂(ICI)的免疫学特征和疗效尚未得到广泛研究[7]。
一项真实世界研究支持这种假设,即ICIs在BRAF突变型NSCLC患者中的疗效可能与在整个NSCLC人群中观察到的疗效相似[8]。
本病例报告旨在描述G466E BRAF突变的NSCLC患者使用帕博丽珠单抗治疗的特点和结局。
该病例对目前已发表的关于携带非V600E BRAF突变的NSCLC的有限文献进行了补充,并提示免疫疗法是一种合理的治疗选择。
我们根据CARE报告清单(https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/rc)来介绍以下病例。
病例展示
患者是一名79岁的男性,因反复出现背痛,于2019年12月进行了诊断性检查。脊柱磁共振成像(MRI)显示脊柱上有多处病理性继发病变。全身计算机断层扫描(CT)显示,左肺下叶(LLL)可见一个至少6 cm的实性结节和胸腔积液,并可见骨转移。
2020年4月,他接受了胸膜活检和胸膜小叶滑石手术,最终诊断为肺腺癌。免疫组化提示程序性死亡配体1(PD−L1)表达为60%,分子检测排除了EGFR、ALK和ROS−1基因的驱动突变的存在,通过二代测序(NGS)进行的综合分子分析确定了G466E BRAF突变。其他与癌症有关的改变是NF1(Y2285fs*5;Y2640fs*3)、TP53(D281Y)。微卫星稳定,肿瘤突变负担(TMB)为5.04 muts/Mb。
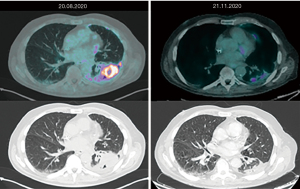
2020年8月进行的正电子发射断层扫描(PET)CT扫描证实为Ⅳ期疾病:胸膜、纵隔结节、肾上腺和骨发现转移病灶。随后,患者开始接受帕博丽珠单抗一线免疫治疗。三个月后,PET−CT扫描显示了良好的治疗效果:肺部和胸膜病变的代谢活跃程度降低、尺寸减小,纵隔结节、肾上腺和骨转移灶则呈现代谢完全缓解(图1)。因此,患者继续使用帕博丽珠单抗治疗。
2021年2月,PET−CT扫描显示胸膜、纵隔淋巴结和第四腰椎的摄取量有轻度升高。然而,鉴于所有其他部位的疾病都取得了临床获益和影像缓解,患者继续接受帕博丽珠单抗治疗。2个月后进行的最后一次PET−CT显示骨呈现代谢完全缓解,肺部、胸膜和肾上腺疾病上则呈现部分代谢缓解。
该患者目前正在继续接受帕博丽珠单抗治疗,并保持着临床获益和良好的安全状况。
本研究中的所有操作及程序都符合本单位和或国家研究委员会的伦理标准以及《赫尔辛基宣言》(2013年修订)。本病例报告及所附图片的发表已获得患者的书面知情同意。书面同意书的副本可供本刊编辑部查阅。由于稿件的非实验性内容,不需要当地伦理委员会的批准。
讨论
BRAF突变是NSCLC的致癌驱动突变之一,它使下游效应分子MEK和ERK磷酸化,以促进细胞增殖和生存。
多个癌种中均涉及MAP激酶通路的改变,因此MEK抑制剂或泛RAF抑制剂或可靶向肺癌中的BRAF突变。此外,MAPK−BRAF联动激活(包括非V600E变体)可能对MEK和ERK的特定信号节点敏感[9]。
目前尚无完整的BRAF突变清单,只有少数文献提到11号外显子和15号外显子的突变[10]。
根据分类系统,BRAF突变被分为三类,G466E BRAF属于III类。
在NSCLC中发现的II~III类突变的临床意义仍不清楚,可能与其他上游MAPK改变有关,如RAS突变或神经纤维瘤病基因(NF1)丢失[11]或功能失活。
III类突变引起的通路改变特异性地通过与野生型C−RAF的二聚化来维持信号。同时,还要求上游激活以增强ERK信号,并通过基因组变异(RAS突变或NF1丢失)或通过受体酪氨酸激酶(RTK)信号获得。这种上游信号传导将对抗ERK介导的对RAS蛋白的负反馈。
III类BRAF突变区别于I类和Ⅱ类的高RAS−GTP水平的反馈[12]。
根据临床实践和病例报告[13],非V600E突变的BRAF/MEK抑制大多在黑色素瘤患者中有重要作用。文献中对维莫非尼(一种BRAF抑制剂)在携带非V600E和V600E BRAF突变的NSCLC患者中的活性略有描述[14]。这类BRAF突变的细胞系中存在的高RAS水平证明了对MEK抑制剂和RTK抑制剂(如EGFR)的组合进行更多的研究是合理的[15,16]。其他研究则基于回顾性数据和病例报告提出ICI的潜在应用价值。Rittberg等人报道了1例罕见的BRAF G469A突变的NSCLC应用纳武单抗成功治疗的病例[17]。Dudnik等人回顾性地分析了BRAF突变的NSCLC患者的PD−L1表达、TMB、微卫星不稳定状态以及对ICI的反应。BRAF突变与高水平的PD−L1表达、低/中度TMB和微卫星稳定状态相关。ICIs在BRAF V600E和BRAF非V600E突变的NSCLC中都显示出良好的活性,ORR分别为25%和33%[18]。同样,Guisier等人认为,ICIs对有可靶向的突变的患者和未选择的人群的疗效相似[19]。作者强调,在44例BRAF突变患者中(其中26例为V600E突变),ORR约为30%(非V600E为33%,V600E突变的患者为26%)。
在NSCLC中,BRAF突变可能比EGFR或ALK改变更接近KRAS突变的影响。事实上,鉴于BRAF与吸烟、PD−L1表达和较高的突变负担有关,与其他致癌驱动因素相比,对ICI的敏感性更高,这是一个生物学原理[20]。
我们介绍了一个非V600E突变的患者对免疫治疗有良好耐受性和反应的例子。这个例子再次显示了驱动基因突变的重要性,以确定最准确的治疗策略,尽管由于缺乏证据,并不总是可能的。事实上,由于NGS的敏感性增加,临床实践中发现了更多意义不明的突变。此外,对有驱动基因突变的NSCLC患者进行免疫治疗,目前尚需大量的证据支持,因此临床决策仍需严格权衡利弊。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine, for the series “Uncommon Mutations in Non-Small Cell Lung Cancer”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-22/coif). The series “Uncommon Mutations in Non-Small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. MR served as the unpaid Guest Editor of the series. MR serves as an unpaid editorial board member of Precision Cancer Medicine from August 2020 to July 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal. Local ethics committee approval was not required due to non-experimental content of the manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen D, Zhang LQ, Huang JF, et al. BRAF mutations in patients with non-small cell lung cancer: a systematic review and meta-analysis. PLoS One 2014;9:e101354. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Marchetti A, Felicioni L, Malatesta S, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol 2011;29:3574-9. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Gautschi O, Milia J, Cabarrou B, et al. Targeted therapy for patients with BRAF-mutant lung cancer: results from the European EURAF cohort. J Thorac Oncol 2015;10:1451-7. [Crossref] [PubMed]
- Lin Q, Zhang H, Ding H, et al. The association between BRAF mutation class and clinical features in BRAF-mutant Chinese non-small cell lung cancer patients. J Transl Med 2019;17:298. [Crossref] [PubMed]
- Blons H, Garinet S, Laurent-Puig P, et al. Molecular markers and prediction of response to immunotherapy in non-small cell lung cancer, an update. J Thorac Dis 2019;11:S25-36. [Crossref] [PubMed]
- Rihawi K, Giannarelli D, Galetta D, et al. BRAF mutant NSCLC and immune checkpoint inhibitors: results from a real-world experience. J Thorac Oncol 2019;14:e57-9. [Crossref] [PubMed]
- Sheikine Y, Pavlick D, Klempner SJ, et al. BRAF in lung cancers: analysis of patient cases reveals recurrent BRAF mutations, fusions, kinase duplications, and concurrent alterations. JCO Precis Oncol 2018;2:PO.17.00172.
- Tissot C, Couraud S, Tanguy R, et al. Clinical characteristics and outcome of patients with lung cancer harboring BRAF mutations. Lung Cancer 2016;91:23-8. [Crossref] [PubMed]
- Dagogo-Jack I, Martinez P, Yeap BY, et al. Impact of BRAF mutation class on disease characteristics and clinical outcomes in BRAF-mutant lung cancer. Clin Cancer Res 2019;25:158-65. [Crossref] [PubMed]
- Frisone D, Friedlaender A, Malapelle U, et al. A BRAF new world. Crit Rev Oncol Hematol 2020;152:103008. [Crossref] [PubMed]
- Dankner M, Lajoie M, Moldoveanu D, et al. Dual MAPK inhibition is an effective therapeutic strategy for a subset of class II BRAF mutant melanomas. Clin Cancer Res 2018;24:6483-94. [Crossref] [PubMed]
- Mazieres J, Montané L, Barlesi F, et al. OA12. 05 Vemurafenib in patients harboring V600 and non V600 BRAF mutations: final results of the NSCLC cohort from the AcSé trial. J Thorac Oncol 2018;13:S348-9. [Crossref]
- Kotani H, Adachi Y, Kitai H, et al. Distinct dependencies on receptor tyrosine kinases in the regulation of MAPK signaling between BRAF V600E and non-V600E mutant lung cancers. Oncogene 2018;37:1775-87. [Crossref] [PubMed]
- Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234-8. [Crossref] [PubMed]
- Rittberg R, Banerji S, Green S, et al. Immunotherapy benefit in a patient with non-small cell lung cancer and a rare BRAF mutation. Cureus 2020;12:e11224. [Crossref] [PubMed]
- Dudnik E, Peled N, Nechushtan H, et al. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol 2018;13:1128-37. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and safety of anti–PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Addeo A, Passaro A, Malapelle U, et al. Immunotherapy in non-small cell lung cancer harbouring driver mutations. Cancer Treat Rev 2021;96:102179. [Crossref] [PubMed]
林偲进
肿瘤学,乳腺外科博士,以第一/共同第一作者发表SCI论文6篇。(更新时间:2023-05-05)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Di Fazio GR, Citarella F, Dell’Aquila E, Russano M, Galletti A, Santo V, Vincenzi B, Tonini G, Santini D. Efficacy of pembrolizumab in lung adenocarcinoma harboring non-V600E BRAF mutation: a case report. Precis Cancer Med 2022;5:10.