Primary lung secretory carcinoma: a case report
Introduction
Salivary gland-like neoplasms are rare tumors that can arise in the lung along the tracheobronchial tree (1). These tumors are thought to originate from the bronchial glands and often produce an endobronchial mass, which can lead to obstructive symptoms. The majority of salivary gland neoplasm arising in this location are either mucoepidermoid or adenoid cystic carcinomas (2). As the classification of salivary gland neoplasms has evolved in recent years, other types of salivary gland neoplasms have been reported in the lung as well. Three reports of secretory carcinoma of the lung have been published: two primary to the lung (3,4), and one metastatic (5). Herein, the authors report a third case of primary secretory carcinoma of the lung.
This case report is written in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/rc).
Case presentation
The patient is a 51-year-old female who presented with worsening shortness of breath and nonproductive cough of three months duration. At the time of initial presentation, imaging revealed a left lung hilar mass measuring 2.2 cm × 1.9 cm × 1.9 cm, with a preoperative biopsy read as a low-grade salivary gland neoplasm. The patient does not smoke tobacco, but does smoke marijuana. She has no significant past medical or surgical history.
A positron emission tomography (PET) scan did not reveal the presence of a mass outside of the lung. Given the tumor’s central location, an open thoracotomy was performed with left pneumonectomy and sampling of N1 and left levels 5 and 6 lymph nodes.
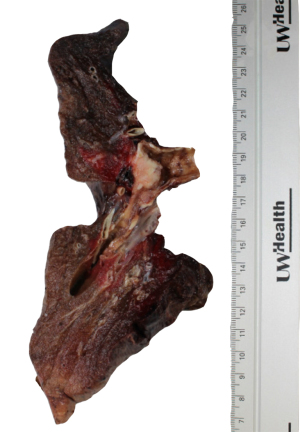
Upon gross examination, the left lung weighed 244 gm and measured 21 cm × 13 cm × 8.5 cm. Sectioning of the lung revealed a 2 cm × 1.8 cm × 1.2 cm endobronchial mass present at the bifurcation of the upper and lower lobar branches. The cut surface of the mass was noted to be pink-white, firm, but slightly granular in texture. Grossly, the mass appeared confined to the bronchus (Figure 1). There was no gross evidence of necrosis. All margins were >1 cm from the mass.
The histologic sections of the tumor revealed solid nests and tubules of tumor cells with focal cyst formation centered on the airway and partially filling the endobronchial space (Figure 2A,2B). The tumor cells showed ample eosinophilic to amphophilic finely vacuolated cytoplasm. The tubules contained brightly eosinophilic to amphophilic, colloid-like secretions (Figure 2C). The tumor nuclei were round to oval and low grade in appearance with only mild pleomorphism. Occasionally prominent nucleoli were identified. Mitotic activity was 3/10 high power fields (HPF). While the tumor did not appear to invade the lung parenchyma, lymphatic invasion was identified, and one hilar lymph node was involved by direct extension.
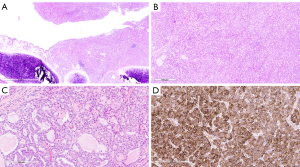
While the tumor morphology was consistent with secretory carcinoma, given the tumor’s location, alternative diagnoses were considered, including a variant of pulmonary adenocarcinoma and an unusual variant of carcinoid tumor. Immunohistochemistry using antibodies raised against S100, p40, TTF-1, synaptophysin, and chromogranin was performed to further characterize the carcinoma. The tumor was diffusely immunoreactive against S100 (Figure 2D), but showed no immunoreactivity against p40, TTF-1, synaptophysin, and chromogranin.
Following the histologic and immunohistochemical analysis of the carcinoma, molecular testing with NTRK gene fusion panel (performed at an outside reference laboratory) that uses next-generation sequencing to identify rearrangements (fusions) involving targeted regions of the NTRK1, NTRK2, and NTRK3 genes was performed, and the ETV6-NTRK3 gene fusion was identified, confirming the diagnosis of secretory carcinoma. The patient did not have an identifiable lesion elsewhere in the body and the secretory carcinoma was concluded to represent a lung primary.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient at the time of surgical consent, for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Salivary gland tumors are rare tumors with an incidence of 1.1 to 1.3 cases per 100,000 in the United States (6). They most commonly occur in the major salivary glands (parotid, submandibular, and sublingual glands), but approximately one quarter (25%) arise from the minor salivary glands throughout the head and neck region (7,8). Salivary gland-like neoplasms, morphologically identical to those described in the major and minor salivary glands, have been described in the lung, and are thought to originate from the bronchial glands lining the tracheobronchial tree. These tumors are far less common than in the salivary glands proper, comprising approximately <1% of all lung tumors (2,9). In contrast to the head and neck region where the benign pleomorphic adenoma predominates, the most commonly encountered salivary gland neoplasms in the lung are mucoepidermoid and adenoid cystic carcinomas, followed by epithelial-myoepithelial carcinoma, and then all others (2). The most recently published WHO Classification of Tumors of the Lung lists only four types of salivary gland-type tumors, mucoepidermoid carcinoma, adenoid cystic carcinoma, epithelial-myoepithelial carcinoma, and pleomorphic adenoma (10). Additional types of salivary gland tumors in the lung are largely restricted to single case reports or very small case series.
The number of different types of salivary gland neoplasms has increased between the time of publishing of the two most recent editions of the WHO Classification of Tumors of the Head and Neck (11,12), incorporating molecular finidngs. Secretory carcinoma is now considered to be a distinct entity which harbors an ETV6-NTRK3 translocation. Secretory carcinoma was originally described in the breast, where it has an excellent prognosis, with a 5-year survival rate approaching 100%, although local recurrences and nodal metastases can occur (13,14). A carcinoma named mammary analogue secretory carcinoma (MASC), was originally reported by Skalova etal. (15), in a series of salivary gland carcinomas with morphologic, immunohistochemical, and genetic characteristics reminiscent of secretory carcinoma of the breast. Most reported cases of MASCs, now termed secretory carcinoma, occur in the parotid gland; however, intraoral minor salivary gland sites can also be involved (16,17). Primary salivary gland secretory carcinoma typically behaves indolently. Primary secretory carcinoma arising outside of the breast and salivary gland, including thyroid gland (18), esophagus (19), skin (20), and vulva (21), have also been reported.
Primary lung secretory carcinoma (3,4) or metastatic secretory carcinoma from salivary gland to the lung is very rare (5). Herein, we report a case of 51-year-old female with primary lung secretory carcinoma, with lymphatic invasion and metastasis to hilar lymph node (10 L). As discussed above, this case demonstrated classical morphologic features of secretory carcinoma, showing a well-circumscribed mass that is composed of microcystic and glandular spaces with abundant eosinophilic secretions, and diffuse expression of S100 in the absence of immunostaning for TTF1, p40, and neuroendocrine markers. This immunohistochemical pattern is consistent with previous reports from Huang et al. (3) and Ramos et al. (4) although additional immunohistochemical stains including mammaglobin were performed in their studies. It has been concluded in the salivary gland, robust co-expression of mammaglobin and S100 proteins confirms the diagnosis of secretory carcinoma without need for molecular studies (22). In particular, mammaglobin has been found to be a very useful marker for differentiating secretory from acinic cell carcinoma, being strongly positive in the former and negative or at most focal weak positive in the latter (22).
In the present case, as morphologic features and immunophenotype of the tumor were consistent with secretory carcinoma, a confirmatory molecular test using next generation sequencing was performed on formalin-fixed paraffin-embedded tissue for NTRK gene fusions. The translocation t(12;15)(p13;q25), in which the ETV6 gene from chromosome 12 is rearranged with the NTRK3 gene from chromosome 15, was identified that confirmed the diagnosis of secretory carcinoma. ETV6-NTRK3 fusion was initially described in infantile fibrosarcoma and congenital mesoblastic nephroma (23), and now it has been associated with in a number of tumor types (24) including acute myeloid leukemia (25), secretory carcinoma (26), and gastrointestinal stromal tumor (27). The biological consequence of this translocation is the expression of a chimeric protein tyrosine kinase with potent transforming activity (28). While reports of some fusion- negative secretory carcinomas exist, the translocation is now believed to be present in almost 100% of true secretory carcinomas (26,29). Over recent years, NTRK fusion-targeted therapy with TRK inhibitors, such as larotrectinib or entrectinib, have proven effective in in vitro and animal studies for treating acute myeloid leukemia (24), and it has been applied to the trial treatment of secretory carcinoma (30).
Including the present case, there are now three reports of primary lung secretory carcinoma. This paucity of cases prevents drawing meaningful conclusions regarding the tumor behavior and clinical prognosis for this entity in this particular location. However, some common features have been observed in the current case and the previous two case reports (3,4). Firstly, all 3 cases occurred in middle age (range 51 to 62 years) females; secondly, both the current case and the one presented by Huang et al. (3) reported local lymph node metastasis; thirdly, increased mitotic activity has been reported in the previous two cases (6 and 10–11/10 HPF), but was lower in the case reported here (3/10 HPF).
In conclusion, primary lung salivary gland tumors are rare, but various types of salivary gland carcinomas can be identified in the lung, including secretory carcinoma. As more cases of primary lung secretory carcinoma are reported, this will hopefully lead to increased knowledge of the clinical behavior of this rare primary lung tumor.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Oncology Tumor Board”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/coif). The series “Precision Oncology Tumor Board” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient at the time of surgical consent for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol 1995;12:106-22. [PubMed]
- Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol 2016;23:13-23. [Crossref] [PubMed]
- Huang T, McHugh JB, Berry GJ, et al. Primary mammary analogue secretory carcinoma of the lung: a case report. Hum Pathol 2018;74:109-13. [Crossref] [PubMed]
- Ramos J, Mahmud W, Ocampo FA, et al. Primary Mammary-Analogue Secretory Carcinoma of the Lung: A Rare Entity With an Unusual Location. Int J Surg Pathol 2020;28:775-81. [Crossref] [PubMed]
- Forner D, Bullock M, Manders D, et al. Secretory carcinoma: the eastern Canadian experience and literature review. J Otolaryngol Head Neck Surg 2018;47:69. [Crossref] [PubMed]
- Lin HH, Limesand KH, Ann DK. Current State of Knowledge on Salivary Gland Cancers. Crit Rev Oncog 2018;23:139-51. [Crossref] [PubMed]
- Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177-84. [Crossref] [PubMed]
- Tian Z, Li L, Wang L, et al. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 2010;39:235-42. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 2015, Lyon (France): IARC Press.
- Barnes LB, Eveson JW, Reichart P, et al. Pathology and genetics of head and neck tumors. 2005, Lyon (France): IARC Press.
- El-Naggar AK, Chan JKC, Grandis JR, et al. WHO classification of head and neck tumours. In: World Health Organization classification of tumours. 4th edition. 2017, Lyon (France): International Agency for Research on Cancer (IARC).
- Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer 1980;45:2404-13. [Crossref] [PubMed]
- Rosen PP, Cranor ML. Secretory carcinoma of the breast. Arch Pathol Lab Med 1991;115:141-4. [PubMed]
- Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34:599-608. [Crossref] [PubMed]
- Chiosea SI, Griffith C, Assaad A, et al. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 2012;61:387-94. [Crossref] [PubMed]
- Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope 2014;124:188-95. [Crossref] [PubMed]
- Dettloff J, Seethala RR, Stevens TM, et al. Mammary Analog Secretory Carcinoma (MASC) Involving the Thyroid Gland: A Report of the First 3 Cases. Head Neck Pathol 2017;11:124-30. [Crossref] [PubMed]
- Chang CY, Chen PH, Hou MC. Esophageal Mammary Analogue Secretory Carcinoma. Clin Gastroenterol Hepatol 2018;16:e11-2. [Crossref] [PubMed]
- Bishop JA, Taube JM, Su A, et al. Secretory Carcinoma of the Skin Harboring ETV6 Gene Fusions: A Cutaneous Analogue to Secretory Carcinomas of the Breast and Salivary Glands. Am J Surg Pathol 2017;41:62-6. [Crossref] [PubMed]
- Nguyen JK, Bridge JA, Joshi C, et al. Primary Mammary Analog Secretory Carcinoma (MASC) of the Vulva With ETV6-NTRK3 Fusion: A Case Report. Int J Gynecol Pathol 2019;38:283-7. [Crossref] [PubMed]
- Mills SE. Chapter 20: Salivary Glands. In: Sternberg’s Diagnostic Surgical Pathology. 6th edition. 2015, Wolters Kluwer Health.
- Adem C, Gisselsson D, Dal Cin P, et al. ETV6 rearrangements in patients with infantile fibrosarcomas and congenital mesoblastic nephromas by fluorescence in situ hybridization. Mod Pathol 2001;14:1246-51. [Crossref] [PubMed]
- Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731-47. [Crossref] [PubMed]
- Joshi SK, Davare MA, Druker BJ, et al. Revisiting NTRKs as an emerging oncogene in hematological malignancies. Leukemia 2019;33:2563-74. [Crossref] [PubMed]
- Skálová A, Vanecek T, Simpson RH, et al. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors With Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am J Surg Pathol 2016;40:3-13. [Crossref] [PubMed]
- Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol 2016;238:543-9. [Crossref] [PubMed]
- Makretsov N, He M, Hayes M, et al. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer 2004;40:152-7. [Crossref] [PubMed]
- Skalova A, Michal M, Simpson RH. Newly described salivary gland tumors. Mod Pathol 2017;30:S27-43. [Crossref] [PubMed]
- Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol 2016;27:920-6. [Crossref] [PubMed]
Cite this article as: Li H, Buehler D, Schulte JJ. Primary lung secretory carcinoma: a case report. Precis Cancer Med 2022;5:7.