一例原发性肺分泌性癌的病例报告
背景介绍
唾液腺样肿瘤是罕见肿瘤,可沿气管支气管树出现在肺内[1]。这些肿瘤被认为起源于支气管腺体,并经常产生支气管内肿块,可能导致阻塞症状。在此位置出现的唾液腺肿瘤大部分是黏液表皮样癌或腺样囊性癌[2]。随着近年来唾液腺肿瘤分类的发展,其他类型的唾液腺肿瘤在肺部也有报道。目前有3例已发表的分泌性肺癌:两例是肺原发[3-4],一例是转移至肺[5]。在此,作者报告了第3例原发性分泌肺癌。
本个案报告根据CARE报告清单撰写(可在https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/rc查阅)。
案例介绍
患者是一名51岁女性,出现持续加重的呼吸困难伴干咳3月。在疾病初期,影像学显示左肺门肿块为2.2 cm×1.9 cm× 1.9 cm,术前活检显示为低级别唾液腺瘤。患者不抽烟,但吸食大麻。无特别既往史及手术史。
正电子发射计算机断层扫描(PET)未发现肺外有肿块。鉴于肿瘤处于中心位置,我们采用开胸手术进行左侧全肺切除术,并对N1及左侧第5、6组淋巴结取样。
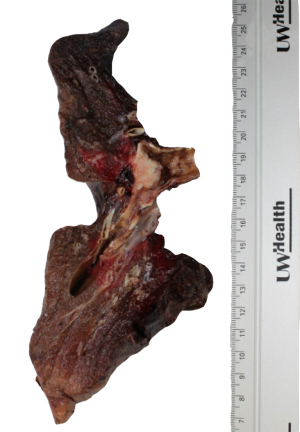
经大体检查,左肺重244 g,测量大小为21 cm×13 cm×8.5 cm。肺切片显示在上下叶分支处有一个2 cm×1.8 cm×1.2 cm的支气管内肿块。肿块的切面呈粉白色,坚固,但质地略呈颗粒状。大体上,肿块局限于支气管内(图1)。没有明显的坏死迹象。所有边缘距肿块>1 cm。
肿瘤组织切片显示肿瘤细胞的实性巢和小管,局灶性囊肿以气道为中心形成,部分充满支气管内腔(图2A-图2B)。肿瘤细胞表现为充足的嗜酸性至嗜两性细胞胞浆。小管内含有明显的嗜酸性至嗜两性的胶质样分泌物(图2C)。肿瘤细胞核呈圆形至椭圆形,外观低度恶性,仅有轻度多形性。偶尔发现明显的核仁。有丝分裂活性为高倍视野(HPF)下3/10。虽然肿瘤没有侵犯肺实质,但发现有淋巴管浸润,一个肺门淋巴结被直接延伸侵犯。
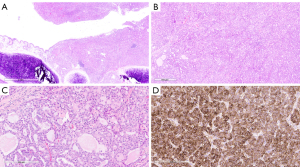
虽然肿瘤形态与分泌性癌一致,但由于肿瘤位置,还是考虑了其他诊断,包括肺腺癌的变异和类癌的异常变异。使用针对S100、p40、TTF-1、突触素和嗜铬粒蛋白的抗体进行免疫组化染色,以进一步表征癌症。肿瘤对S100呈弥漫性免疫反应(图2D),但对p40、TTF-1、突触素和嗜铬粒蛋白没有免疫反应。
经过对肿瘤的组织学和免疫组化分析后,使用二代测序靶向NTRK1、NTRK2和NTRK3基因对NTRK基因融合进行分子检测。我们在肿瘤中鉴定到ETV6-NTRK3基因融合,从而确认其分泌性癌的诊断。因患者身体其他部位没有可识别的病变,分泌性癌被认为是原发性肺癌。
本研究中执行的所有程序均符合机构和国家研究委员会的道德标准以及赫尔辛基宣言(2013年修订)。书面知情同意书是在手术同意的时候从患者处获得的,用于出版这份手稿和任何附带的图像。书面同意书的副本可供本刊编辑部审阅。
讨论
唾液腺肿瘤是一种罕见肿瘤,在美国的发病率为每10万人中有1.1~1.3例[6]。它们通常发生在主要的唾液腺(腮腺、下颌下腺和舌下腺),但大约四分之一(25%)来自整个头颈部的小唾液腺[7-8]。形态与大、小唾液腺类似的唾液腺样肿瘤已在肺中有报道,被认为是起源于排列在气管支气管树上的支气管腺。这些肿瘤远不如唾液腺本身常见,约占所有肺部肿瘤的1%[2,9]。与以良性多形性腺瘤为主的头颈部区域相反,肺中最常见的唾液腺肿瘤是黏液表皮样和腺样囊性癌,其次是上皮−肌上皮癌,然后是其他所有类型的肿瘤[2]。最近出版的世界卫生组织肺部肿瘤分类仅列出4种类型的唾液腺型肿瘤,包括黏液表皮样癌、腺样囊状癌、上皮−肌上皮癌和多形性腺瘤。其他类型的唾液腺肿瘤在肺部主要限于单一病例报告或非常小的病例系列。
在世界卫生组织头颈部肿瘤分类[11-12]的两个最新版本之间,不同类型的唾液腺肿瘤的数量有所增加,纳入了分子发现。分泌性癌现在被认为是一个独特的实体,其包含ETV6-NTRK3易位。分泌性癌最初在乳腺中被描述,其预后良好,5年生存率接近100%,尽管可能发生局部复发和淋巴结转移[13-14]。一种名为乳腺类似物分泌性癌(MASC)的肿瘤最初由Skalova等人报道[15],在一系列具有形态学、免疫组化和遗传特征的唾液腺癌中,使人联想到乳腺分泌性癌。大多数报道的MASCs,现在称为分泌性癌,主要发生在腮腺;然而也可能涉及口内小唾液腺[16,17]。原发性唾液腺分泌性癌通常表现为惰性。乳腺和唾液腺以外的原发性分泌性癌,包括甲状腺[18]、食道[19]、皮肤[20]和外阴[21]也有报道。
原发性肺分泌性癌[3-4]或从唾液腺转移到肺的分泌性癌是罕见的[5]。在此,我们报告一例51岁女性原发性肺分泌性癌,淋巴管浸润并转移至肺门淋巴结(10L)。如上所述,该病例显示出分泌性癌的经典形态学特征,表现为由具有丰富嗜酸性分泌物的微囊和腺体间隙所组成的边界清晰的肿块,以及在TTF1、p40和神经内分泌标志物不表达情况下S100的弥漫性表达。这种免疫组化模式与Huang等人[3]和Ramos等人[4]以前的报道一致,尽管在他们的研究中进行了包括乳腺珠蛋白在内的其他免疫组化染色。结果表明,在唾液腺中,乳腺珠蛋白和S100蛋白的稳定共表达证实了分泌性癌的诊断,而不需要进行分子研究[22]。特别是,乳腺珠蛋白是鉴别分泌性癌和腺泡细胞癌的一个非常有用的标志物,前者为强阳性,后者为阴性或最多为局灶性弱阳性[22]。
在本例中,由于肿瘤的形态学特征和免疫表型与分泌性癌一致,所以使用二代测序在福尔马林固定的石蜡包埋组织上进行NTRK基因融合的验证性分子检测。t(12;15)(p13;q25)易位突变的鉴定,即来自12号染色体的ETV6基因与来自15号染色体的NTRK3基因的重排,证实了分泌性癌的诊断。ETV6-NTRK3融合最初在婴儿纤维肉瘤和先天性中胚层肾瘤中发现[23],现已与多种肿瘤类型[24]有关,包括急性骨髓性白血病[25]、分泌性癌[26]和胃肠道基质肿瘤[27]。这种易位的生物学后果是具有强大转化活性的融合蛋白质酪氨酸激酶的表达[28]。虽然有一些融合阴性的分泌性癌报道,但是这种易位突变现在被认为是存在于几乎100%的真性分泌性癌[26,29]。近年来,用TRK抑制剂(如larotrectinib或entrectinib)进行的NTRK融合靶向治疗已被证明在体外和动物研究中对治疗急性骨髓性白血病有效[24],并已应用于分泌性癌的临床试验中[30]。
包括本例在内,目前已有3例原发性肺分泌性癌的报道。由于缺乏病例,因此无法就这一特定部位的肿瘤行为和临床预后得出有意义的结论。然而,在本病例和前两个病例报告[3-4]中已观察到一些共同的特征。首先,这3例患者均发生在中年(范围51~62岁)女性;其次,目前的病例和黄等人[3]的报告均有局部淋巴结转移;第三,有丝分裂活性在前两个病例中比较活跃(6和10−11/10 HPF),但在本病例中较低(3/10 HPF)。
综上所述,原发性肺涎腺肿瘤罕见,但包括分泌性癌在内的各种类型涎腺癌均可在肺内被鉴别。随着越来越多的原发性肺分泌性癌病例被报道,将有望加深这种罕见的原发性肺肿瘤的临床表现的认识。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “Precision Oncology Tumor Board”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-15/coif). The series “Precision Oncology Tumor Board” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient at the time of surgical consent for publication of this manuscript and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Moran CA. Primary salivary gland-type tumors of the lung. Semin Diagn Pathol 1995;12:106-22. [PubMed]
- Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol 2016;23:13-23. [Crossref] [PubMed]
- Huang T, McHugh JB, Berry GJ, et al. Primary mammary analogue secretory carcinoma of the lung: a case report. Hum Pathol 2018;74:109-13. [Crossref] [PubMed]
- Ramos J, Mahmud W, Ocampo FA, et al. Primary Mammary-Analogue Secretory Carcinoma of the Lung: A Rare Entity With an Unusual Location. Int J Surg Pathol 2020;28:775-81. [Crossref] [PubMed]
- Forner D, Bullock M, Manders D, et al. Secretory carcinoma: the eastern Canadian experience and literature review. J Otolaryngol Head Neck Surg 2018;47:69. [Crossref] [PubMed]
- Lin HH, Limesand KH, Ann DK. Current State of Knowledge on Salivary Gland Cancers. Crit Rev Oncog 2018;23:139-51. [Crossref] [PubMed]
- Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg 1986;8:177-84. [Crossref] [PubMed]
- Tian Z, Li L, Wang L, et al. Salivary gland neoplasms in oral and maxillofacial regions: a 23-year retrospective study of 6982 cases in an eastern Chinese population. Int J Oral Maxillofac Surg 2010;39:235-42. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 2015, Lyon (France): IARC Press.
- Barnes LB, Eveson JW, Reichart P, et al. Pathology and genetics of head and neck tumors. 2005, Lyon (France): IARC Press.
- El-Naggar AK, Chan JKC, Grandis JR, et al. WHO classification of head and neck tumours. In: World Health Organization classification of tumours. 4th edition. 2017, Lyon (France): International Agency for Research on Cancer (IARC).
- Tavassoli FA, Norris HJ. Secretory carcinoma of the breast. Cancer 1980;45:2404-13. [Crossref] [PubMed]
- Rosen PP, Cranor ML. Secretory carcinoma of the breast. Arch Pathol Lab Med 1991;115:141-4. [PubMed]
- Skálová A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol 2010;34:599-608. [Crossref] [PubMed]
- Chiosea SI, Griffith C, Assaad A, et al. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology 2012;61:387-94. [Crossref] [PubMed]
- Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope 2014;124:188-95. [Crossref] [PubMed]
- Dettloff J, Seethala RR, Stevens TM, et al. Mammary Analog Secretory Carcinoma (MASC) Involving the Thyroid Gland: A Report of the First 3 Cases. Head Neck Pathol 2017;11:124-30. [Crossref] [PubMed]
- Chang CY, Chen PH, Hou MC. Esophageal Mammary Analogue Secretory Carcinoma. Clin Gastroenterol Hepatol 2018;16:e11-2. [Crossref] [PubMed]
- Bishop JA, Taube JM, Su A, et al. Secretory Carcinoma of the Skin Harboring ETV6 Gene Fusions: A Cutaneous Analogue to Secretory Carcinomas of the Breast and Salivary Glands. Am J Surg Pathol 2017;41:62-6. [Crossref] [PubMed]
- Nguyen JK, Bridge JA, Joshi C, et al. Primary Mammary Analog Secretory Carcinoma (MASC) of the Vulva With ETV6-NTRK3 Fusion: A Case Report. Int J Gynecol Pathol 2019;38:283-7. [Crossref] [PubMed]
- Mills SE. Chapter 20: Salivary Glands. In: Sternberg’s Diagnostic Surgical Pathology. 6th edition. 2015, Wolters Kluwer Health.
- Adem C, Gisselsson D, Dal Cin P, et al. ETV6 rearrangements in patients with infantile fibrosarcomas and congenital mesoblastic nephromas by fluorescence in situ hybridization. Mod Pathol 2001;14:1246-51. [Crossref] [PubMed]
- Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731-47. [Crossref] [PubMed]
- Joshi SK, Davare MA, Druker BJ, et al. Revisiting NTRKs as an emerging oncogene in hematological malignancies. Leukemia 2019;33:2563-74. [Crossref] [PubMed]
- Skálová A, Vanecek T, Simpson RH, et al. Mammary Analogue Secretory Carcinoma of Salivary Glands: Molecular Analysis of 25 ETV6 Gene Rearranged Tumors With Lack of Detection of Classical ETV6-NTRK3 Fusion Transcript by Standard RT-PCR: Report of 4 Cases Harboring ETV6-X Gene Fusion. Am J Surg Pathol 2016;40:3-13. [Crossref] [PubMed]
- Brenca M, Rossi S, Polano M, et al. Transcriptome sequencing identifies ETV6-NTRK3 as a gene fusion involved in GIST. J Pathol 2016;238:543-9. [Crossref] [PubMed]
- Makretsov N, He M, Hayes M, et al. A fluorescence in situ hybridization study of ETV6-NTRK3 fusion gene in secretory breast carcinoma. Genes Chromosomes Cancer 2004;40:152-7. [Crossref] [PubMed]
- Skalova A, Michal M, Simpson RH. Newly described salivary gland tumors. Mod Pathol 2017;30:S27-43. [Crossref] [PubMed]
- Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC). Ann Oncol 2016;27:920-6. [Crossref] [PubMed]

葛丽萍
女,中共党员,目前于复旦大学附属肿瘤医院攻读博士,导师邵志敏教授。硕博连读期间以第一作者及共同一作在Molecular Cancer, Oncogene, Breast Cancer Research and Treatment等发表SCI四篇。在读期间,获复旦大学一等奖学金、复旦大学优秀学生干部、复旦大学优秀学生及复旦大学优秀团员等荣誉。(更新时间:2023-05-05)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Li H, Buehler D, Schulte JJ. Primary lung secretory carcinoma: a case report. Precis Cancer Med 2022;5:7.