Differences in optimal timing of post-surgical surveillance for limited stage lung cancer patients and associations with outcomes
Introduction
Lung cancer is the second most common cancer in both men and women, with estimates of over 230,000 new cases expected in 2018 (American Cancer Society). Of the cases of lung cancer in the US, 85% are non-small cell lung cancer (NSCLC). The standard of care for early stage lung cancer is surgery, however, the guidelines for post-operative surveillance is variable. Historically, surgeons have been using a one-size fits all approach, such that there is little incorporation of important prognostic indicators for recurrence and survival reflected in the current surveillance guidelines.
Rice et al. reported that for all patients who have undergone resection for NSCLC the risk for a second primary lung cancer is 1–4% per patient-year (1). Therefore follow-up of these patients is important, but also begs the question on the best way to follow them. In 2013 Mollberg and Ferguson published a review on the topic of post-resection surveillance and called for a more patient centered algorithm for surveillance after resection (2).
Most recent National Comprehensive Cancer Network (NCCN) guidelines have recommended scans to be performed every 6 months for 2 to 3 years then annually thereafter. This is a more intense surveillance regimen than that suggested by the recent findings of the Intergroupe Francophone de Cancerologie Thoracique (IFCT-0302) Trial which was a randomized controlled study comparing an intensive surveillance CT regimen to follow-up with routine clinic visits and chest X-ray alone (3). There was no difference in overall survival comparing regimens and the authors suggested that CT scans done every 6 months are not useful at all for early stage patients within the first 2 years following surgery.
Our study examined patient follow-up CT scans, the timing in which the scans occurred, and when documented recurrences where detected in order to help determine optimal timing for detection of recurrence by CT scan. We also sought to determine whether there was an association between timing of CT surveillance and overall survival.
Methods
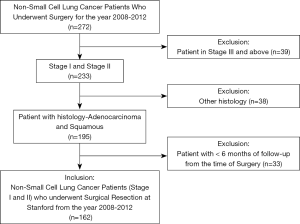
The Institutional Review Board of Stanford University approved this study. The study design was a retrospective review of patients who underwent surgical resection for lung cancer at Stanford Hospital between the years 2008–2012. Data was pulled from our institutional Society of Thoracic Surgeons (STS) Thoracic Surgery Database. A total of 272 patients were identified. Chart review from our own electronic medical record was used for data collection. We excluded patients stage III and above (n=39), those who had histology other than adenocarcinoma and squamous (n=38), and patients with less than six months of follow-up from the time of surgery (n=33). The final study population consisted of 162 patients (Figure 1). All patients were staged according to the AJCC 7th edition lung cancer staging.
Our primary outcome was recurrence and secondary outcome was receipt of CT scan reviewed at Stanford during the surveillance period. We reviewed all imaging and progress notes for the primary clinical endpoints. Adhering to NCCN guidelines for lung cancer management, recommended chest CT surveillance was defined as receipt of CT scan at 180–210 days (month 6) following date of surgical resection. Chest CT every six months for first-two years following surgery and once a year thereafter were used as the standard follow-up guidelines. Each CT scan was further coded by the indication for the study and study findings. If a lesion was documented in the patient’s chart as recurrence by either the treating oncologist or thoracic surgeon, then it was deemed a recurrence in our data set. CT-detected recurrence was defined either as a new radiographic finding on CT scan in conjunction with biopsy confirmation or documentation by the patient’s medical oncologist as definite recurrence. Clinical recurrence was defined as any documented recurrence whether or not it was detected by CT scan or other means. Other recurrences were either detected by imaging being performed for symptoms, non-lung cancer related reasons such as trauma, or recurrences noted clinically by the cancer center registrar for which there was no accompanying surveillance imaging test.
Statistics
Continuous data were compared as mean and standard deviation, median and inter quartile range (IQR), and categorical variables as frequency and percentage. Tests of Normality of the continuous data was performed using Kolmogorov-Smirnov test. Patient demographics were compared using independent-sample t-test and ANOVA test for continuous variables and Pearson’s Chi-square test for categorical variables. Time to event analyses was performed using Kaplan-Meier method and the differences were tested using log-rank test. Independent predictors of risk of recurrences and death risk were estimated using cox proportional hazard model. A probability (P) value of <0.05 was considered to be statistically significant. Statistical analyses were completed using SAS® Enterprise Guide (EG) 7.1 (SAS Institute, Cary, NC, USA) and IBM SPSS Statistics 24.
Results
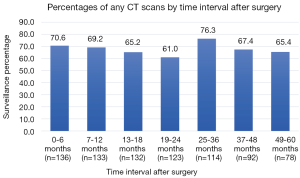
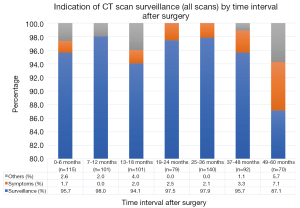
The cohort consisted of 80% stage I and 20% stage II patients with median follow-up 57 months (IQR 24.75) (Table 1). Adherence to guideline recommended surveillance ranged from 61–76.3% (Figure 2) with the indication for the majority all CT scans done being for surveillance purposes (87–98%) (Figure 3). Recurrence occurred in 27.2% of patients at a median of 29.5 months following surgery. On univariate analysis, only stage was associated with recurrence such that 20.8% of stage I patients had documented recurrence vs. 53.1% of the stage II patients (P<0.01) (Table 2). Higher stage was found to be a significant risk for recurrence even after adjusting for comorbidities and other tumor characteristics [HR 4.31 (95% CI, 2.17–8.58) P<0.01] (Table 3).
Table 1
| Patient characteristics | N=162 (%) |
|---|---|
| Gender | |
| Male | 67 (41.4) |
| Female | 95 (58.6) |
| Age (median/IQR) | 70/11 |
| Race | |
| Caucasian | 101 (62.3) |
| Asian | 37 (22.8) |
| Other | 24 (14.8) |
| Smoking status | |
| Never smoked | 54 (33.3) |
| Smoked | 108 (66.7) |
| Cumulative Charlson comorbidities | |
| 0 | 95 (58.6) |
| 1 | 38 (23.5) |
| ≥2 | 29 (17.9) |
| Stage/prognostics group | |
| Stage I | 130 (80.2) |
| Stage II | 32 (19.8) |
| Resection type | |
| Lobar | 122 (75.3) |
| Sublobar | 39 (24.1) |
| Pneumonectomy | 1 (0.6) |
| Grade differentiation | |
| Well | 50 (30.9) |
| Moderate | 75 (46.3) |
| Poor | 31 (19.1) |
| Cannot be assessed | 6 (3.7) |
| Pleural invasion | 28 (17.3) |
| Lymphovascular invasion | 8 (4.9) |
| Mutation status | |
| EGFR positive (n=101) | 32 (31.7) |
| KRAS positive (n=86) | 19 (22.1) |
| ALK positive (n=72) | 6 (8.3) |
| Triple negative (n=65) | 29 (44.6) |
| Adjuvant therapy | |
| Adjuvant chemotherapy | 22 (13.6) |
| Adjuvant radiation | 4 (2.5) |
| Recurrence (yes) | 44 (27.2) |
| Median time to recurrence months/IQR | 29.5/26 |
| Median follow-up months/IQR | 56.5/24.75 |
| Vital status | |
| Dead | 29 (17.9) |
Table 2
| Patient characteristics | Recurrence (no =118), n (%) | Recurrence (yes =44), n (%) | P value |
|---|---|---|---|
| Gender | 0.32 | ||
| Male | 46 (39.0) | 21 (47.7) | |
| Female | 72 (61.0) | 23 (52.3) | |
| Age (median/IQR) | 69/15 | 70/11 | 0.99 |
| Race | 0.08 | ||
| Caucasian | 76 (64.4) | 25 (56.8) | |
| Asian | 22 (18.6) | 15 (34.1) | |
| Others | 20 (16.9) | 4 (9.1) | |
| Smoking status | 0.62 | ||
| Never smoked | 38 (32.2) | 16 (36.4) | |
| Smoked | 80 (67.8) | 28 (63.6) | |
| Cumulative Charlson comorbidities | 0.28 | ||
| 0 | 73 (61.9) | 22 (50.0) | |
| 1 | 27 (22.9) | 11 (25.0) | |
| ≥2 | 18 (15.3) | 11 (25.0) | |
| Stage/prognostics group | <0.01* | ||
| Stage I | 103 (87.3) | 27 (61.4) | |
| Stage II | 15 (12.7) | 17 (38.6) | |
| Resection type | 0.16 | ||
| Lobar | 87 (73.7) | 35 (79.5) | |
| Sublobar | 31 (26.3) | 8 (18.2) | |
| Grade differentiation | 0.13 | ||
| Well | 41 (34.7) | 9 (20.5) | |
| Moderate | 48 (40.7) | 27 (61.4) | |
| Poor | 24 (20.3) | 7 (15.9) | |
| Cannot be assessed | 5 (4.2) | 1 (2.3) | |
| Pleural invasion | 17 (14.4) | 11 (25.0) | 0.11 |
| Lymphovascular invasion | 5 (4.2) | 3 (6.8) | 0.50 |
| Mutation status | |||
| EGFR positive (n=101) | 19 (28.4) | 13 (38.2) | 0.31 |
| KRAS positive (n=86) | 11 (19.6) | 8 (26.7) | 0.45 |
| ALK positive (n=72) | 2 (4.5) | 4 (14.3) | 0.20 |
| Triple negative (n=65) | 22 (53.7) | 7 (29.2) | 0.06 |
| Adjuvant therapy | |||
| Adjuvant chemotherapy | 13 (11.0) | 9 (20.5) | 0.12 |
| Adjuvant radiation | 2 (1.7) | 2 (4.5) | 0.30 |
| Median follow-up months (IQR) | 59 | 56.50 | 0.61 |
*, P≤0.05. NSCLC, non-small cell lung cancer.
Table 3
| Important predictors | Risk for recurrences | Recurrence-EGFR tested | Recurrence-KRAS tested | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |||
| Race | |||||||||||
| Asian vs. Caucasian | 1.75 | 0.90–3.41 | 0.10 | 1.87 | 0.85–4.14 | 0.12 | 3.11 | 1.26–7.66 | 0.01* | ||
| Others vs. Caucasian | 0.54 | 0.18–1.65 | 0.28 | 0.12 | 0.02–1.02 | 0.05* | 0.50 | 0.06–3.98 | 0.51 | ||
| Cumulative Charlson comorbidities score | |||||||||||
| 1 vs. 0 | 1.78 | 0.82–3.87 | 0.15 | 2.63 | 1.04–6.61 | 0.04* | 3.20 | 1.16–8.84 | 0.03* | ||
| ≥2 vs. 0 | 2.08 | 0.96–4.48 | 0.06 | 2.10 | 0.86–5.14 | 0.11 | 1.95 | 0.71–5.41 | 0.20 | ||
| Stage | |||||||||||
| II vs I | 4.31 | 2.17–8.58 | <0.01* | 3.75 | 1.67–8.40 | <0.01* | 5.04 | 2.11–12.1 | <0.01* | ||
| Chemo adjuvant therapy status | |||||||||||
| Yes vs. no | 1.30 | 0.59–2.87 | 0.52 | 2.82 | 1.06–7.49 | 0.04* | 2.29 | 0.83–6.36 | 0.11 | ||
| EGFR mutation status | |||||||||||
| Yes vs. no | 1.00 | 0.43–2.32 | 0.99 | ||||||||
| KRAS mutation status | |||||||||||
| Yes vs. no | 1.59 | 0.62–4.08 | 0.33 | ||||||||
*, P≤0.05. NSCLC, non-small cell lung cancer.
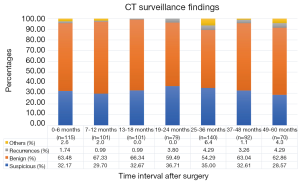
The timing of recurrences also differed significantly based on stage such that the majority, 81% (n=27) of recurrences presenting more than 24 months following surgery for stage I patients compared to 41% (n=17) of recurrences in stage II patients (P<0.01). The rate of suspicious findings on CT scans remained relatively stable over time ranging from 30–35% (Figure 4). However, the rate of CT scans with confirmed recurrence was variable, peaking at 2–3 years following surgery for the entire cohort (Figure 5). Also for the entire cohort, the time at which CT recurrences were detected varied with 36% (n=7) identified less than 24 months after surgery while the majority of all recurrences, 63% (n=12) were detected more than 24 months after surgery. When examined by stage, however stage I recurrences peaked at 25–36 months (Figure 6) compared to stage II patients with an earlier recurrence peak at 19–24 months (Figure 7) (P<0.01). When looking at overall recurrences, stage was a significant predictor of for time to recurrence. For stage I patients 75.9% recurred after 2 years, whereas stage II patients recurred earlier with 66.7% of their recurrences happening before two years (P<0.01) (Table 4). Recurrences occurred in 7 squamous cell patients, 35 adenocarcinoma patients, and 2 patients with adenosquamous histology (Table 4). Overall, higher rates of surveillance CT were associated with a reduced risk of death [HR 0.14 (95% CI, 0.06–0.36) P<0.01].
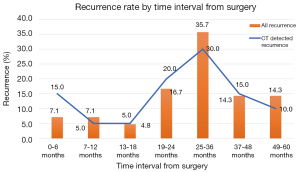
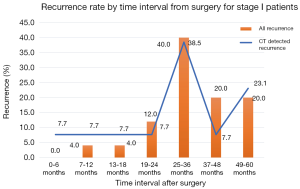
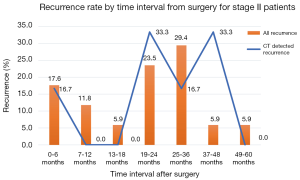
Table 4
| Characteristics | Staging, n (%) | Histopathologic type, n (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Stage I | Stage II | P | Squamous cell carcinoma | Adenocarcinoma | Adenosquamous carcinoma | P | ||
| CT detected recurrences (n=19) | ||||||||
| <24 months (n=7) | 4 (57.1) | 3 (42.9) | 0.62 | 2 (28.6) | 5 (71.4) | 0 (0.0) | 1.00 | |
| >24 months (n=12) | 9 (75.0) | 3 (25.0) | 3 (25.0) | 8 (66.7) | 1 (8.3) | |||
| All recurrences (n=44) | ||||||||
| <24 months (n=15) | 5 (33.3) | 10 (66.7) | ≤0.01* | 1 (6.7) | 13 (86.7) | 1 (6.7) | 0.46 | |
| >24 months (n=29) | 22 (75.9) | 7 (24.1) | 6 (20.7) | 22 (75.9) | 1 (3.4) | |||
*, P≤0.05.
Discussion
We know that not all cancers are biologically equal, yet surveillance strategies remain the same for all patients. Clinical and pathologic stage remain some of the strongest predictors of outcomes in lung cancer and even in the earliest stage patients, recurrence is problematic. Rubins and colleagues, reported post-resection recurrence rates for Stage I tumors to be anywhere from 20–39% (4). More recently Pepek and colleagues at Duke University reported their five-year rates of locoregional recurrence to be 16%, 26%, 43%, 35%, and 40% for stages IA, IB, IIB, and IIIA disease respectively (5). Furthermore, the risk of recurrence is cumulative increasing over time and is compounded by the risk of developing metachronous tumors estimated at 1–2% per year (6).
Following surgery for curative intent, lung cancer patients are generally followed post-operatively with surveillance scans which are dictated by NCCN guidelines. Historically, these guidelines have been uniform, applying the same surveillance strategy to all post-operative patients. The newest iteration of the NCCN guidelines are tailored according to pathologic stage and type of treatment received (7). In this version, the panel now recommends more frequent (i.e., more “intense”) surveillance with CT every 3–6 months for patients with late cancer stage (stage III or IV) or treated with radiation therapy compared to those with early cancer stage (stages I and II) or treated with chemotherapy. The new guidelines have reasonable clinical basis given higher rates of recurrence associated with late stage disease. Nonetheless, there has been no new high-level evidence to support the change. Historically, limited single institution or single time point studies have been used to support certain surveillance strategies and outcomes. A retrospective study of 1,294 lung cancer patients undergoing surveillance CT imaging found 60% of recurrences in asymptomatic patients with a false-positive rate of 25% (8). Another study found that 78% of recurrences detected during surveillance were in asymptomatic patients (9). Both authors concluded that recurrent disease is rarely missed by post treatment surveillance CT and recommended its routine use.
The recommendations for more intense surveillance for late stage patients are in contrast to the recent findings of the IFCT which recommend less frequent surveillance for late stage patients based on their findings of a lack of survival benefit in this group. The IFCT data did suggest that more intense surveillance could be beneficial for certain subgroup populations, namely: males, patients with early stage disease, and those having undergone surgery as sole treatment. More recently, a large national study conducted by McMurry and colleagues among 4,463 stage I–III patients examined surveillance CT scans and also found that more frequent surveillance was not associated with longer risk-adjusted overall survival nor with post-recurrence survival (10).
To our knowledge, ours is the first large study examining recurrence as it relates to timing of surveillance in an attempt to move towards a more tailored approach to care for lung cancer patients. We found the rate of recurrence to be 27.5% of all early staged lung cancer patients at a median of 29.5 months following surgery which is on par with reported rates of recurrence in the literature. Martini and colleagues looked at stage I lung cancer patients and found the overall incidence of recurrence for resected lung cancer to be 27%, with 60% of those recurrences occurring within two years of the initial operation (11). Varlotto and colleagues reported local recurrence rates for patients with potentially curative resection for stage I NSCLC at 2, 3, and 5 years to be 14%, 21%, and 29% respectively (12). Similar to other published reports, we found stage to be the strongest predictor of recurrence in this select cohort. Thus, if stage is indeed the strongest predictor, our findings that the timing of recurrence differed significantly according to stage make sense and that stage should be considered when defining surveillance strategies aimed at reducing unnecessary procedures and cost.
While the percentage of CT scans with suspicious findings was relatively stable over time in our study, the rate of CT scans with documented recurrence was variable. We found that the peak time for recurrence to be 2–3 years following surgery. When we broke down stage I and stage II, we found that stage I recurrences peaked slightly later at 25–36 months whereas stage II peaked slightly earlier at 19–24 months. Once again, this data shows that factoring stage into a surveillance strategy would be prudent.
There are limitations to our study. First, due to the time frame in which our study took place, we found that many of the early patients of a specific surgeon in the cohort were not followed up with CT scans but rather CXR in the early post-resection years. Also, due to the fact that we are a tertiary center with referrals from significant distances, we had a significant amount of people who did not receive follow-up with us and therefore were lost to follow up unless referred back to us for a recurrence, potentially confounding the findings. Our follow up is limited as well as we had to choose a starting point and end point to include patients. Although it is short, we chose the minimum follow up of 6 months in order to limit the main analyses to those patients for whom surveillance is appropriate. The maximal follow up is only limited by the fact that we were following NCCN guidelines for 5 years.
In conclusion, as we continue to work in an arena where we are able to get more and more information on each individual cancer, whether it be the stage, histological type, genetic mutational status, or any other variable, we should work to incorporate this information into a more patient specific surveillance regimen to optimize our use of CT scans and better serve our patients. Our data suggests that the timing of recurrence differs significantly based on stage such that few stage I patients have recurrences within 2 years following surgical resection, however these patients currently get scans every 6 months in the first two years. Although more investigation is warranted, our data may help support recommending less frequent scans in the early post-operative period. Optimal timing of CT surveillance based on peak recurrence rates has the potential to eliminate unnecessary testing and expense for healthcare systems.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.10.02). LB reports personal fees from Johnson and Johnson, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study has been approved by an institutional review board at Stanford University. Protocol #39411. This study will not affect the future management of the patients. Informed consent was not obtained for this retrospective study as information was taken through chart review. The patient’s personal data was secured using REDCAP.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg 2003;76:1001-7; discussion 1007-8. [Crossref] [PubMed]
- Mollberg NM, Ferguson MK. Postoperative surveillance for non-small cell lung cancer resected with curative intent: developing a patient-centered approach. Ann Thorac Surg 2013;95:1112-21. [Crossref] [PubMed]
- Westeel V, Barlesi F, Foucher P, et al. Results of the phase III IFCT-0302 trial assessing minimal versus CT-scan-based follow-up for completely resected non-small cell lung cancer (NSCLC). Ann Oncol 2017;28: Issue suppl_5.
- Rubins J, Unger M, Colice GL; American College of Chest Physicians. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest. 2007;132:355S-367S.
- Pepek JM, Chino JP, Marks LB, et al. How well does the new lung cancer staging system predict for local/regional recurrence after surgery?: A comparison of the TNM 6 and 7 systems. J Thorac Oncol 2011;6:757-61. [Crossref] [PubMed]
- Van Meerbeeck J, Weyler J, Thibaut A, et al. Second primary lung cancer in Flanders: frequency, clinical presentation, treatment and prognosis. Lung Cancer 1996;15:281-95. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 6.2018. 2018 Aug 17. National Comprehensive Cancer Network. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81; discussion 81-2. [Crossref] [PubMed]
- Korst RJ, Kansler AL, Port JL, et al. Accuracy of surveillance computed tomography in detecting recurrent or new primary lung cancer in patients with completely resected lung cancer. Ann Thorac Surg 2006;82:1009-15; discussion 1015. [Crossref] [PubMed]
- McMurry TL, Stukenborg GJ, Kessler LG, et al. More Frequent Surveillance Following Lung Cancer Resection Is Not Associated With Improved Survival: A Nationally Representative Cohort Study. Ann Surg 2018;268:632-9. [Crossref] [PubMed]
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg 1995;109:120-9. [Crossref] [PubMed]
- Varlotto JM, Recht A, Flickinger JC, et al. Varying recurrence rates and risk factors associated with different definitions of local recurrence in patients with surgically resected, stage I nonsmall cell lung cancer. Cancer 2010;116:2390-400. [PubMed]
Cite this article as: Colwell E, Benson J, Bhandari P, He H, Shrager J, Lui N, Berry M, Backhus L. Differences in optimal timing of post-surgical surveillance for limited stage lung cancer patients and associations with outcomes. Precis Cancer Med 2019;2:31.