
Direct comparison of autofluorescence bronchoscopy (AFB) and the combination of autofluorescence bronchoscopy and white light bronchoscopy (AFB + WLB) for detecting airway cancerous and precancerous lesions: a systematic review and meta-analysis
Introduction
Bronchoscopy has become an effective tool for detecting airway cancer and even precancerous lesions. In addition to conventional white light bronchoscopy (WLB), nowadays there are several advanced techniques, such as autofluorescence bronchoscopy (AFB). Compared to WLB, AFB presented higher sensitivity, better overall performance but lower specificity for lung cancer and precancerous lesions in several meta-analyses (1-4). Given the diagnostic properties of AFB and WLB (AFB: high sensitivity but low specificity; WLB: low sensitivity but high specificity), combining WLB with AFB (AFB + WLB) may be one of the feasible strategies for improving cancer detection. For that, a superior overall performance (including sensitivity) of AFB + WLB has been proved compared to WLB alone (4). However, whether AFB + WLB also has a better overall performance than AFB alone is still lack of sufficient evidence. Therefore, in this article we conducted a systematic review and meta-analysis, directly comparing the sensitivity, specificity and overall diagnostic performance between AFB and AFB + WLB.
Methods
This article was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (5). Study selection (Jianrong Zhang blind with Yujing Yang, Hua Liao, Ziyan Liang, Xuewei Chen, Minzhang Guo), quality assessment (Jianrong Zhang blind with Jieyu Wu) and data extraction (Jianrong Zhang blind with Jieyu Wu, Zhiheng Xu, Yujing Yang, Hua Liao, Ziyan Liang) was done independently by authors. Any discrepancies were resolved by discussion with Wenhua Liang.
Database retrieval and study selection
PubMed and Web of Science were searched from the inception date of each database to 31 Dec 2017. The retrieval formula was: ((Fluorescence OR Autofluorescence OR Autofluorescence Imaging) AND Bronchoscopy) AND Cancer [Human][English]. We also searched eligible articles from the database of our previous published research, which was conducted based on the similar formula in PubMed, Web of Science, Scopus, Embase, ProQuest (scholarly journals), the Cochrane Library and Ovid (all EBM review), according to the inception dates of these seven databases to Mar 20, 2015 (4).
Comparative studies regarding AFB versus AFB + WLB for diagnosing lung cancer and/or precancerous lesions were eligible, and data should be sufficient for constructing a 2×2 contingency table with histopathology as the reference standard. In our study, AFB + WLB was defined as the diagnostic procedure that was conducted by both AFB and WLB; both consecutive and simultaneous procedures were eligible. Duplicated articles were deleted and articles with inappropriate types of publication were excluded, such as reviews, systematic reviews, meta-analyses, case reports, letters and comments. Articles with fewer details and/or worse quality were also excluded if two articles overlapped with the same/similar authors, institutions, study periods or relevant data.
Quality assessment
We assessed all included studies based on the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (6). Question 3 in domain 4 of QUADAS-2, “Were all patients included in the analysis?” was replaced with “Were all patients/biopsy specimens included in the analysis?” because there are two types of analysis for constructing 2×2 tables (patient-based or biopsy-based analysis). Based on the results, we rated the quality of each study according to the following criteria: “low risk” and “low concern” in all domains indicate high quality; “high risk”, “high concern”, “unclear risk” or “unclear concern” indicates moderate or low quality).
Data extraction
Characteristics of included articles were extracted, including author, year, total patients for analysis, type of analysis, histopathological findings, AFB technique, sensitivity and specificity of AFB and AFB + WLB. Only the data we extracted were used in the final statistical analysis of each individual study; for example, the number of patients enrolled in studies would not be equal to the number of patients finally analyzed. In this case, we extracted the data from the second situation.
We calculated true positive (TP), false positive (FP), false negative (FN) and true negative (TN) for 2×2 tables according to the given data from included studies and corresponding formulas (7,8). In detail, TP was considered when samples were detected as positive by bronchoscopy and were also confirmed as positive by histopathology. FP, FN as well as TN were positive in bronchoscopy but negative in histopathology, negative in bronchoscopy but positive in histopathology, as well as negative in both bronchoscopy and histopathology, respectively. One kind of comparative articles was excluded during this process: the total number of lesions (TP + FP + FN + TN) or their positive/negative results (TP + FN/FP + TN) was not equal when the performance of AFB versus AFB + WLB was investigated.
Statistical analysis
We used a bivariate random-effect model to estimate the pooled sensitivity, specificity, diagnostic odds ratio (DOR) and the area under the summary receiver operating characteristic curve (AUC) with 95% confidence interval (95% CI). We also plotted the hierarchical summary receiver-operating characteristic (HSROC) curve for the overall performance. A subgroup analysis was made based on the pathological diagnostic criteria.
A meta-regression was conducted to access the effect of potential covariates for heterogeneity, such as period, analysis type and study quality. The p value and the I2 index for the heterogeneity analysis were based on a joint model, which considered sensitivity and specificity simultaneously during meta-regression. If heterogeneity was indicated (P<0.05 or I2>50%), we made a subgroup analysis to compare the sensitivity and specificity of AFB and AFB + WLB. We did not estimate the publication bias for there is no existing test perfectly matching this type of meta-analysis (9).
All pooling procedures were conducted in software STATA 13.0 (StataCorp, College Station, TX, USA) with the midas and metandi commands. We also used software Meta-DiSc 1.4 (XI Cochrane Colloquium, Barcelona, Spain) if the study number of groups or subgroup elements was only three.
Results
Study identification is showed in Figure 1. Seven comparative studies were finally included, involving a total of 904 patients and 2,740 biopsy specimens (10-16). The details of study characteristics and quality assessment were demonstrated in Tables 1 and S1.

Table 1
| Author & Year | Patient (n) | Biopsy (n) | Analysis type | Positive finding of histopathology | AFB technique | AFB | AFB + WLB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Total | MIL | MOD | SEV | CIS | INV | Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | ||||||
| Venmans 1999 (10) | 95 | 660 | Biopsy-based | MOD->CIS | 79 | – | 31 | 39 | 9 | – | LIFE | 80 | 62 | 85 | 60 | |
| Kusunoki 2000 (11) | 65 | 216 | Biopsy-based | SEV->INV | 49 | – | – | 21 | 9 | 19 | LIFE | 86 | 89 | 90 | 78 | |
| Hirsch 2001 (12) | 55 | 391 | Biopsy-based | ASD | 71 | 71 | – | – | LIFE | 75 | 46 | 90 | 23 | |||
| Fuso 2005 (13) | 166 | 166 | Biopsy-based | MOD->INV | 93 | – | 13 | 80 | D-light | 91 | 51 | 100 | 44 | |||
| Ernst 2005 (14) | 293 | 821 | Biopsy-based | MOD->INV | 85 | – | 85 | D-light | 61 | 75 | 66 | 73 | ||||
| Herth 2009 (15) | 62 | 98 | Patient-based | MOD->CIS | 17 | – | 17 | – | AFI | 65 | 40 | 65 | 35 | |||
| Divisi 2010 (16) | 168 | 388 | Biopsy-based | MOD->INV | 328 | – | 328 | SAFE-3000 | 100 | 60 | 96 | 60 | ||||
The data we extracted was only responsible for the final statistical analysis of each individual study. MIL, mild dysplasia; MOD, moderate dysplasia; SEV, severe dysplasia; CIS, carcinoma in situ; INV, invasive carcinoma; ASD, angiogenic squamous dysplasia; AFB, Autofluorescence bronchoscopy; WLB, white light bronchoscopy.
Our included studies involved four different categories of AFB technique: LIFE, D-light, AFI and SAFE-3000. Except the absolute differences of the sensitivities between AFB and AFB + WLB were 15% and 9% in two studies, the differences in other four studies were within 5%. Regardless of which categories was investigated, no specificities of AFB + WLB were higher than the specificities of AFB according to the original reported data (Table 1).
The results of meta-analysis are presented in Table 2. The overall sensitivity, DOR and AUC of AFB + WLB were close to those of AFB, but the overall specificity of AFB + WLB was lower (Figure 2). Similar situation is shown in subgroup analysis based on histopathological diagnostic criteria.
Table 2
| Group/subgroup | Study (n) | Patient (n) | Biopsy (n) | Technique | Sensitivity (%) | Specificity (%) | DOR | AUC (%) |
|---|---|---|---|---|---|---|---|---|
| Summary | 7 | 904 | 2,740 | AFB | 88 [65–97] | 63 [49–75] | 12 [3–54] | 77 [73–81] |
| AFB + WLB | 90 [77–96] | 54 [39–68] | 11 [4–34] | 78 [74–81] | ||||
| INV->SEV | 1 | 65 | 216 | AFB | 86 [50–100] | 89 [80–97] | – | – |
| AFB + WLB | 91 [62–100] | 79 [58–99] | – | – | ||||
| INV->MOD | 3 | 627 | 1,375 | AFB | 95 [86–100] | 63 [45–81] | 19 [3–110] | 75 [52–97] |
| AFB + WLB | 96 [87–100] | 60 [40–79] | 23 [4–143] | 78 [72–83] | ||||
| CIS->MOD | 2 | 157 | 758 | AFB | 74 [34–100] | 52 [30–75] | – | – |
| AFB + WLB | 77 [43–100] | 49 [23–74] | – | – | ||||
| ASD | 1 | 55 | 391 | AFB | 75 [20–100] | 46 [17–75] | – | – |
| AFB + WLB | 91 [63–100] | 23 [7–39] | – | – |
Data in square brackets are 95% CIs. DOR, diagnostic odds ratio; AUC, area under the receiver operating curve; AFB, autofluorescence bronchoscopy; WLB, white light bronchoscopy.
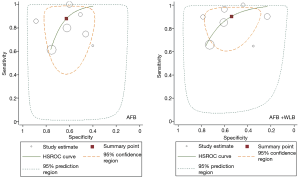
The assessment for heterogeneity is shown in Table S2. Based on the result, study quality was indicated as the source of heterogeneity; accordingly, we conducted another subgroup analysis based on study quality. In high-quality studies, the sensitivity of AFB was lower than that of AFB + WLB, but an opposite result was shown in moderate & low studies. Compared with AFB, AFB + WLB presented lower specificity regardless of high or moderate & low studies (Table S3).
Discussion
To our knowledge, this systematic review and meta-analysis is the first to compare the diagnostic performance between AFB and AFB + WLB for lung cancer and precancerous lesions. We have showed that AFB and AFB + WLB presented similar overall sensitivity, specificity and overall diagnostic performance (DOR and AUC). However, the specificity of AFB + WLB was lower than that of AFB regardless of overall comparison and the comparison in subgroup analysis.
Based on our findings, we are wondering why AFB + WLB cannot improve the specificity and the overall performance of AFB, since the high specificity of WLB has been proved for cancer detection (1-4). We assume that the increased sensitivity (after combination with WLB) could cause more false positive results, which lowers the specificity to some extent.
As we know, adding WLB during the AFB procedure could lengthen the time of the procedure and increase the number of biopsy specimens, which may potentially raise the risk of bronchoscopic operation as well as the rate of injury to patients. Considering the lower specificity of AFB + WLB, as well as the similar sensitivity, DOR and AUC of AFB and AFB + WLB in our study, whether using AFB alone is enough for detecting lung cancer and precancerous lesions needs to be further discussed.
With respect to the low specificity of AFB + WLB, another combination—AFB combined with narrow-band imaging bronchoscopy (AFB + NBI)—has been investigated and its sensitivity, specificity, DOR and AUC were 86% (95% CI: 82–89%), 75% (71–79%), 28 [3–257] and 96% (standard error 0.05), respectively, for pre-malignant lesions in a meta-analysis (17). This remarkable property can be explained by the diagnostic performance of NBI: over 80% sensitivity and specificity when NBI alone was used (17). Therefore, NBI alone or its combination with WLB, AFB or other techniques shows a promising prospect for airway cancer diagnosis.
Some limitation should be considered in this study. Firstly, we tried to conduct the database by searching as comprehensively as possible in order to include more eligible studies, but the number of studies was still insufficient for calculating DOR and AUC in some elements of subgroups. In addition, we set histopathological diagnostic criteria for subgroup analysis to investigate whether both techniques can cover different types of lesions during diagnosis. This does not mean that AFB or AFB + WLB can distinguish the histopathological degree of detected lesions. The confirmation of these detected lesions should be finally conducted in histopathology. Thirdly, we were unable to recognize the bronchoscopic diagnostic criteria of each included study. This condition could be a reason for the heterogeneity in our meta-analysis, which attenuates the confidence of our study.
Table S1
| Author & year | Risk of bias | Applicability concerns | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1Q1 | D1Q2 | D1Q3 | D1 | D2Q1 | D2Q2 | D2 | D3Q1 | D3Q2 | D3 | D4Q1 | D4Q2 | D4Q3 | D4 | D1 | D2 | D3 | ||
| Venmans 1999 (10) | U | Y | U | U | Y | U | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Kusunoki 2000 (11) | U | Y | U | U | Y | Y | L | Y | U | L | U | Y | Y | L | U | L | L | |
| Hirsch 2001 (12) | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Fuso 2005 (13) | Y | Y | U | L | Y | Y | L | Y | Y | L | U | Y | Y | L | L | L | L | |
| Ernst 2005 (14) | N | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Herth 2009 (15) | U | Y | Y | L | Y | Y | L | Y | Y | L | U | Y | N | U | L | L | L | |
| Divisi 2010 (16) | U | Y | U | U | Y | Y | L | Y | U | L | U | N | Y | U | U | L | L | |
Risk of bias: D1 = Domain 1, patient selection; D2 = Domain 2, index test; D3 = Domain 3, reference standard; D4 = Domain 4, flow and timing; D1Q1 = was a consecutive or random sample of patients enrolled? D1Q2 = was a case-control design avoided? D1Q3 = did the study avoid inappropriate exclusions? D2Q1 = were the index test results interpreted without knowledge of the results of the reference standard? D2Q2 = if a threshold was used, was it prespecified? D3Q1 = is the reference standard likely to correctly classify the target condition? D3Q2 = were the reference standard results interpreted without knowledge of the results of the index test? D4Q1 = was there an appropriate interval between the index test and reference standard? D4Q2 = did all patients receive the same reference standard? D4Q3 = were all patients or biopsy specimens included in the analysis? Applicability concern: D1 = Domain 1, are there concerns that the included patients and setting do not match the review question? D2 = Domain 2, are there concerns that the index test, its conduct, or its interpretation differ from the review question? D3 = Domain 3, are there concerns that the target condition as defined by the reference standard does not match the question? Y, yes; N, no; U, unclear; H, high; L, low.
Table S2
| Covariate | Study (n) | Patient (n) | Biopsy (n) | AFB | AFB + WLB | |||
|---|---|---|---|---|---|---|---|---|
| P (Joint) | I2 | P (Joint) | I2 | |||||
| Time | 0.560 | 0% (0–100) | 0.970 | 0% (0–100) | ||||
| 2004–2010 | 4 | 689 | 1,473 | |||||
| 1999–2003 | 3 | 215 | 1,267 | |||||
| Quality | 0.360 | 2% (0–100) | 0.010 | 78% [51–100] | ||||
| High quality | 2 | 221 | 557 | |||||
| Moderate & low quality | 5 | 683 | 2,183 | |||||
| Analysis | 0.290 | 20% (0–100) | 0.170 | 44% (0–100) | ||||
| Biopsy-based analysis | 6 | 842 | 2,642 | |||||
| Patient-based analysis | 1 | 62 | 98 | |||||
P (Joint), P value based on joint model, which has considered sensitivity and specificity together to estimate whether heterogeneity exists (P<0.05); I2, I2 index: I2≤25% = low heterogeneity, I2>25% and ≤50% = moderate heterogeneity, I2>50% = high heterogeneity. AFB, autofluorescence bronchoscopy; WLB, white light bronchoscopy.
Table S3
| Quality | Study (n) | Patient (n) | Biopsy (n) | Technique | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| High quality | 2 | 221 | 557 | AFB | 85 [54–100] | 48 [26–71] |
| AFB + WLB | 98 [93–100] | 32 [15–50] | ||||
| Moderate & low quality | 4 | 683 | 2,183 | AFB | 89 [73–100] | 68 [56–81] |
| AFB + WLB | 85 [71–98] | 64 [52–75] |
Acknowledgments
The author thanks Ms. Carolyn Smith (Senior Tutor, The Writing Center at Washington University in St. Louis) for providing suggestions on manuscript revision. This research was presented at ESMO Asia 2015 Congress.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2018.06.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The institutional ethical approval was waived. Because of the retrospective nature of the research, the informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: a meta-analysis. Lung Cancer 2011;73:183-8. [Crossref] [PubMed]
- Sun J, Garfield DH, Lam B, et al. The value of autofluorescence bronchoscopy combined with white light bronchoscopy compared with white light alone in the diagnosis of intraepithelial neoplasia and invasive lung cancer: a meta-analysis. J Thorac Oncol 2011;6:1336-44. [Crossref] [PubMed]
- Wang Y, Wang Q, Feng J, et al. Comparison of autofluorescence imaging bronchoscopy and white light bronchoscopy for detection of lung cancers and precancerous lesions. Patient Prefer Adherence 2013;7:621-31. [PubMed]
- Zhang J, Wu J, Yang Y, et al. White light, autofluorescence and narrow-band imaging bronchoscopy for diagnosing airway pre-cancerous and early cancer lesions: a systematic review and meta-analysis. J Thorac Dis 2016;8:3205-16. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [Crossref] [PubMed]
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. [Crossref] [PubMed]
- Altman DG, Bland JM. Diagnostic tests. 1: Sensitivity and specificity. BMJ 1994;308:1552. [Crossref] [PubMed]
- Altman DG, Bland JM. Diagnostic tests 2: Predictive values. BMJ 1994;309:102. [Crossref] [PubMed]
- Leeflang MM. Systematic reviews and meta-analyses of diagnostic test accuracy. Clin Microbiol Infect 2014;20:105-13. [Crossref] [PubMed]
- Venmans BJ, Van Boxem TJ, Smit EF, et al. Results of two years expenience with fluorescence bronchoscopy in detection of preinvasive bronchial neoplasia. Diagn Ther Endosc 1999;5:77-84. [Crossref] [PubMed]
- Kusunoki Y, Imamura F, Uda H, et al. Early detection of lung cancer with laser-induced fluorescence endoscopy and spectrofluorometry. Chest 2000;118:1776-82. [Crossref] [PubMed]
- Hirsch FR, Prindiville SA, Miller YE, et al. Fluorescence versus white-light bronchoscopy for detection of preneoplastic lesions: a randomized study. J Natl Cancer Inst 2001;93:1385-91. [Crossref] [PubMed]
- Fuso L, Pagliari G, Boniello V, et al. Autofluorescence bronchoscopy to identify pre-cancerous bronchial lesions. Monaldi Arch Chest Dis 2005;63:124-8. [Crossref] [PubMed]
- Ernst A, Simoff MJ, Mathur PN, et al. D-light autofluorescence in the detection of premalignant airway changes: a multicenter trial. J Bronchology Interv Pulmonol 2005;133-8.
- Herth FJ, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060-5. [Crossref] [PubMed]
- Divisi D, Di Tommaso S, De Vico A, et al. Early diagnosis of lung cancer using a SAFE-3000 autofluorescence bronchoscopy. Interact Cardiovasc Thorac Surg 2010;11:740-4. [Crossref] [PubMed]
- Iftikhar IH, Musani AI. Narrow-band imaging bronchoscopy in the detection of premalignant airway lesions: a meta-analysis of diagnostic test accuracy. Ther Adv Respir Dis 2015;9:207-16. [Crossref] [PubMed]
Cite this article as: Zhang J, Wu J, Xu Z, Yang Y, Liao H, Liang Z, Jiang L, Li J, Guo M, Chen X, Zeng Y, He Q, Liang W, He J. Direct comparison of autofluorescence bronchoscopy (AFB) and the combination of autofluorescence bronchoscopy and white light bronchoscopy (AFB + WLB) for detecting airway cancerous and precancerous lesions: a systematic review and meta-analysis. Precis Cancer Med 2018;1:5.

