
Current and future perspectives in clinical practice in KRAS-mutated non-small cell lung cancer: a literature review
Introduction
The histopathological classification of non-small cell lung cancer (NSCLC) has given way in recent years to a molecular classification with major therapeutic implications (1). This is largely due to the routine implementation of PD-L1 expression analysis and the emergence of next-generation sequencing (NGS) that allow, at a low cost, the molecular characterisation of tumours showing that, within the same histological subtype, there are large differences in biological behaviour due to the different genes that may be affected (2). Due to these advances, it has been possible to develop different therapies against genomic alterations in NSCLC that have allowed a change in the natural history of these tumours (3), with response rates and patient survival rates that were unimaginable a decade ago (4,5).
Specific treatments that have been approved as first-line regimens against different mutations such as those targeting the EGFR, ALK, ROS1, KRAS, BRAF, RET or MET genes are commercially available (6,7). These mutations occur in approximately 10% of lung cancers in Europe and the United States and in up to 35% of cases diagnosed in Asian countries in a profile of younger patients who are light smokers or never smoked and with a histological diagnosis of adenocarcinoma (8) (Figure 1). Alterations in other genes such as ALK, ROS1 and BRAF are less frequent, appearing individually in approximately 3–7%, 2% and 1% of patients with NSCLC (9,10). Of these, the most frequent are mutations in the KRAS oncogene, which are strongly associated with smoking and may appear in around 20–30% of lung adenocarcinomas, with residual occurrence in the squamous and undifferentiated subtype (11).
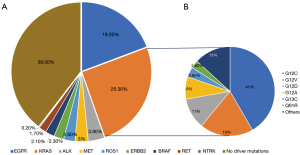
A key point in the advancement of treatments in NSCLC with target mutations is to understand the influence that these genes can have on the response to immunotherapy (12). Alterations in the EGFR gene are increasingly known to be resistant to immunotherapy, and treatment with immune checkpoint inhibitors is not very effective in terms of response and survival compared to tumours without mutations in this gene (13). For the other genes the implications are not known, however, in the case of the KRAS gene, the data are highly contradictory, although in recent years they seem to suggest a greater response (14). Some studies indicate a greater effect of immunotherapy in KRAS-mutated NSCLC such as the research published by Amanam et al. or Peng et al. (15,16). Likewise, the study by Lauko et al. in patients with KRAS-mutated NSCLC and brain involvement also seems to indicate a good effect of immunotherapy (17). However, other studies do not seem to show a greater effect of immunotherapy such as those of Justeau et al. or Sciortino et al. (18,19).
For all these reasons, mutations in the KRAS gene have multiple implications in NSCLC, both at the level of the new drugs available that target these mutations and that are allowing action on KRAS to increase patient survival, as well as the effect of these mutations on immunotherapy or chemo-immunotherapy treatments. The aim of this research is to review the role of mutations in the KRAS gene in NSCLC both in terms of their influence on the response to immunotherapy and in the response to new drugs that target KRAS. We present this article in accordance with the Narrative Review reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-26/rc).
Methods
Search strategy
The narrative review was conducted and carried out using the quality standards of narrative or literature review current guidelines (Table 1). The reviews, clinical trials and studies included were found through searching several databases (2010–2023): PubMed, Cochrane, Science Direct, EMBASE, and the clinical trial registry (www.clinicaltrials.gov).
Table 1
| Items | Specification |
|---|---|
| Date of search | May to December 2023 |
| Databases and other sources searched | PubMed, Cochrane, Science Direct, EMBASE, and clinicaltrials.gov |
| Search terms used | “Lung cancer OR lung OR NSCLC”, “KRAS inhibitors OR KRAS G12C inhibitors”, “Immunotherapy OR immune checkpoint inhibitors OR ICI” and “clinical trials OR approved drugs OR review”. Several combinations were used to search the databases: [“(Lung Cancer OR NSCLC) “AND” (KRAS inhibitors OR KRAS G12C inhibitors) “AND” (Immunotherapy OR immune checkpoint inhibitors OR ICI)” AND/OR “(clinical trials OR approved drugs OR review)”] |
| Timeframe | 2010–2023 |
| Inclusion criteria | The studies reviewing the main clinical trials that resulted in the approval of targeted therapies in KRAS-mutated NSCLC and then the immunotherapy treatment in this type of lung cancer. Subsequently, existing literature reviews were selected, as well as studies that involved data analysis from different hospitals. Abstracts that, due to their novelty or importance, influenced the oncology field were also included |
| Selection process | Two independent reviewers (L.P.D. and M.M.G.) completed the data extraction and examination. Following this, a third reviewer (A.O.H.) compared the results and assessed the consistency of the results with the current literature like a systematic review |
Various text and medical subject headings (MeSH) combinations were used during the search: “Lung cancer OR lung OR NSCLC”, “KRAS inhibitors OR KRAS G12C inhibitors”, “Immunotherapy OR immune checkpoint inhibitors OR ICI” and “clinical trials OR approved drugs OR review”. Several combinations were used to search the databases: [“(Lung Cancer OR NSCLC) “AND” (KRAS inhibitors OR KRAS G12C inhibitors) “AND” (Immunotherapy OR immune checkpoint inhibitors OR ICI)” AND/OR “(clinical trials OR approved drugs OR review)”]. All studies that provided current evidence on the present or future treatment of NSCLC KRAS G12C mutated were considered.
Study selection and data extraction
Initially, the studies reviewing the main clinical trials that resulted in the approval of targeted therapies in KRAS-mutated NSCLC and then the immunotherapy treatment in this type of lung cancer. Subsequently, existing literature reviews were selected, as well as studies that involved data analysis from different hospitals. Abstracts that, due to their novelty or importance, influenced the oncology field were also included.
KRAS and tumour microenvironment (TME)
There is evidence that mutations in KRAS induce a pro-inflammatory state in the TME that accelerates tumour development. Overexpression of KRAS increases the secretion of interleukin-6 (IL-6), which is involved in the early stages of tumour development. IL-6 promotes the activation of Janus-activated kinase 1 (JAK1) and the phosphorylation of transcription factor 3 (STAT3). In addition, studies have shown that IL-6 activates reactive oxygen species (ROS) through the ERK89 pathway promoting inflammation in the TME. IL-8 is also related to tumourigenesis induced by this oncogene as it is involved in signal transduction through the ERK or phosphatidylinositol 3-kinase (PI3K) pathways mediated by KRAS favouring endothelial cell recruitment, tumour-associated inflammation and angiogenesis. IL-8 is highly implicated in NSCLC progression through the IL-8/CXCR2 pathway that contributes to neutrophil recruitment and neutrophil elastase release in the TME. This pathway also induces the generation of cancer-associated fibroblasts that have enhanced pro-tumour cytokine secretory function. Several studies have shown that KRAS mutations induce an increase in cell adhesion molecule 1 (CAM 1) favouring the recruitment of M1 macrophages which, through the release of cell matrix degrading enzymes such as metalloprotease 9 and cytokines such as tumour necrosis factor (TNF), contribute to a pro-inflammatory environment. In the TME of patients with KRAS mutations, elevated levels of IL-17 have been found to be associated with neutrophil recruitment and tumour progression (20,21).
Within the TME, the influence of KRAS favours tumour escape by promoting PD-L1 expression and decreasing MHC-I expression in tumour cells, hindering the adhesion of leukocytes with antitumour activity. In summary, mutations in KRAS generally promote a pro-inflammatory tumour environment, accelerate tumour growth and dissemination, and facilitate tumour escape from host immunity (22).
Development of targeted therapies for KRAS
The KRAS gene, located at locus 12p12.1, is one of the three homologues of the RAS family, together with H-RAS and N-RAS, one of the most frequently altered groups of oncogenes in human cancers. Of the three, KRAS is the most commonly altered member and its involvement in pancreatic adenocarcinoma stands out, where it is mutated in approximately 65–95% of cases. Other tumours in which it appears are colon adenocarcinoma (35% of cases), cholangiocarcinoma (12–14%), endometrial carcinoma (12–14%), testicular germ cell tumours (10–12%) or ovarian serous carcinoma (<10% of cases) (23,24). In NSCLC, KRAS mutations occur in 20–30% of cases (25).
KRAS encodes a small G protein of the GTPase subtype (guanosine triphosphate hydrolase activity) which is bound to the cell membrane. When this protein is bound to GDP it is inactive and when it is bound to GTP it is activated inducing signal transduction leading to the activation of different cell signalling pathways involved in proliferation. Its activation is favoured by guanine nucleotide exchange factors (GEF) such as SOS1/2. Their inactivation by GTP hydrolysis is favoured by GTPase activating proteins (GAP) such as neurofibromin 1 (NF1) (26). The balance between hydrolysis and exchange determines the levels of active KRAS in cells.
KRAS activates different effector proteins that control functions involved in the cell cycle. The most studied pathways include RAF kinases and the catalytic subunit of PI3K. Binding of KRAS-GTP to RAF kinases stimulates their dimerisation and activation, triggering consecutive activation of MEK and ERK and provoking cell cycle progression. When bound to PI3K, KRAS mediates the activation of AKT and mTOR regulating processes involved in cell metabolism and apoptosis among other (27,28).
Mutations that occur in KRAS are single amino acid substitutions that activate an oncoprotein that prevents GTP hydrolysis leading to hyperactivation of the pathway. Substitutions at codons 12 or 13 prevent the hydrolysis process by a critical arginine residue in GAPs. Other less frequent variants such as Q61H/L/R/K interfere with the coordination of the water molecule involved in hydrolysis (29).
Establishing a targeted therapy against KRAS mutations has historically been a very complicated challenge due to multiple circumstances. The protein encoded by the KRAS oncogene has a high intrinsic affinity for GTP, making it complex to disrupt these bindings. This affinity has made it difficult to develop small molecules that can effectively compete with GTP for binding to KRAS. Traditionally, KRAS oncoproteins were thought to be permanently activated. However, pre-clinical studies show that they can switch between active and inactive states in tumour cells (2,30). This variation between active and inactive states makes it difficult to develop specific inhibitors that permanently block their activity. There are several mutations described in the KRAS oncogene, each of which modifies the protein, giving it a different structure. As the final protein has a different conformation, each mutation in this oncogene will require a targeted therapy, making the development of a single therapy targeting all mutations inconceivable.
KRAS mutations in NSCLC
In NSCLC, the most frequent KRAS mutations are found at codon 12, exon 2. These mutations involve an amino acid substitution that activates the oncoprotein. KRAS with the substitution of glycine (G) for cysteine (C) at codon 12 (G12C). It is the most common mutation accounting for approximately 40% of KRAS mutations in NSCLC and occurs in approximately 10–15% of lung adenocarcinomas and is largely associated with heavy smokers (31). In second and third place, with a much lower incidence, are alterations in G12 V, replacement of G by valine (V) at position 12, also closely associated with smoking, and G12D, replacement of G by aspartic acid (D) at codon 12, this mutation appears more frequently in non-smokers.
In codon 13, the most frequently occurring mutation involves the substitution of G for C, G13C. Finally, mutations have also been documented at codon 61, most notably the Q61H mutation, substitution of glutamic acid (Q) for histidine. KRAS point mutations at codons 12, 13 and 61 account for 98% of the total number of alterations described in this oncogene (31).
Different studies have shown that there is biochemical heterogeneity of KRAS mutations, each providing a different intrinsic GTPase activity, as demonstrated by the difference in activity between G12D, G12C and G13D, for example. Within the G12C mutation, it is worth noting that it has been associated with a different rate of hydrolysis than other mutations. This allows it to switch from the predominantly GTP-bound (active) form to the GDP-bound (inactive) form, at which point it is vulnerable to the action of covalent KRAS inhibitors such as sotorasib (32). This conformational change and the fact that there are times when the protein is insensitive to the action of targeted therapies makes the search for a specific treatment for this mutation complicated.
Thus, within each type of tumour, each of the different mutations provides a different distant dissemination profile. In general, patients with KRAS mutations are more likely to have lung or brain metastases. In those with G12V mutations, pleural and pericardial metastases are common, while patients with G12C and G12D mutations frequently have bone involvement (33).
Influence of KRAS comutations on the response to therapies in NSCLC
One of the features that has traditionally hindered the development of targeted therapies against KRAS is the frequent association of comutations in other genes related to tumour growth (Figure 2). A study in patients with metastatic NSCLC and mutated KRAS found comutations in 53% of patients (34). Of these, TP53 mutations were most prominent, accounting for 39%. Other mutations with a lower frequency were those that appeared in the gene encoding serine-threonine kinase 11 (STK1) and the KEAP gene (kelch-like ECH associated protein 1). Other studies have shown that, despite a higher frequency, comutations in the gene encoding serine/threonine kinase (ATM) and cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) are also important (35).
In contrast to the traditional thinking that EGFR and KRAS mutations were mutually exclusive in NSCLC, studies have shown that, although at a very low frequency of 1–4%, they can occur (13,29). In the subgroup of NSCLC patients with KRAS and TP5 3 involvement, an increased inflammatory response and activation of JAK-STAT and interferon-mediated pathways is detected. In addition, the expression of co-stimulatory molecules such as CD-28 and immune response inhibitors such as PD-L1 is also increased. On the other hand, the rate of megabase alterations is also higher than in other types of comutations (36). On the other hand, in those cases in which KRAS and STK11/LKB1 comutation is detected, the TME shows little infiltration by CD8+ cytotoxic lymphocytes, which favours greater immunosenescence. Finally, in tumours with KRAS and CDKN2A/B comutation, tumour cells show increased resistance to oxidative stress and targeted therapies, thanks in part to the development of hypoxia-inducible factor 1-alpha. In summary, in NSCLC, the presence of comutations together with KRAS mutations can have a profound impact on disease behaviour and response to treatment (37). Knowing which comutations appear most frequently is essential in daily clinical practice to adapt the therapeutic strategy as shown in the following section.
KRAS inhibitors in clinical practice for NSCLC
In recent years, the identification of a novel allosteric binding site dependent on covalent cysteine inhibition in KRAS G12C has allowed targeted therapies acting as irreversible covalent inhibitors to be developed in the last five years and have proven their efficacy in daily clinical practice. However, such strategies are not very useful against other highly prevalent mutations such as KRAS G12D and KRAS G12V. For these mutations, some compounds have been discovered with promising results in in-vitro and in-vivo assays, but are not yet expected to be marketed.
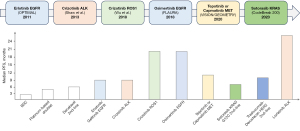
The KRAS inhibitors currently approved by the Food And Drug Administration (FDA) for use in clinical practice are sotorasib and adagrasib, both of which act as inhibitors of the G12C mutation (Table 2). The CodeBreak 100 clinical trial was a phase 1/2 study evaluating the efficacy of sotorasib in KRAS G12C-mutated solid tumours, including patients with advanced or metastatic NSCLC. Among all patients in the phase 2 study, which enrolled 126 patients (81% previously treated with chemotherapy and/or immunotherapy), the objective response rate (ORR) was 37.1% [95% confidence interval (CI): 28.6–46.2%] with a complete response rate of 3.2% and a partial response rate of 33.9%. Progression-free survival (PFS) was 6.8 months (95% CI: 5.1–8.2) with an overall survival (OS) of 12.5 months (95% CI: 10 to could not be evaluated). The adverse event rate was 69.8%, with a grade 3 event rate of 19.8% (38,39). The main adverse events were gastrointestinal and hepatic.
Table 2
| Characteristics | CODEBREAK 100 phase II | CODEBREAK 200 (phase III) | KRYSTAL-I (phase I–II) | |||
|---|---|---|---|---|---|---|
| Sotorasib | Sotorasib | Docetaxel | Adagrasib | |||
| N | 126 | 171 | 174 | 116 | ||
| Median age [range], years | 63.5 [37–80] | 64.0 [32–88] | 64.0 [35–87] | 64 [25–89] | ||
| Sex, n (%) | ||||||
| Female | 63 (50) | 62 (36.3) | 79 (45.4) | 65 (56) | ||
| Male | 63 (50) | 109 (63.7) | 95 (54.6) | 51 (44) | ||
| PD-L1 protein expression, n (%) | ||||||
| <1% | 33 (26) | 57 (33.3) | 55 (31.6) | 47 (40.5) | ||
| ≥1–49% | 30 (24) | 46 (26.9) | 70 (40.2) | 27 (23.3) | ||
| ≥50% | 35 (28) | 60 (35.1) | 40 (23.0) | 12 (10.3) | ||
| Desconocido | 28 (22) | 8 (4.7) | 9 (5.2) | 30 (25.9) | ||
| Disease stage, n (%) | ||||||
| Locally advanced | 5 (4.0) | 9 (5.3) | 8 (4.6) | 13 (11.2) | ||
| Metastatic | 121 (96.0) | 162 (94.7) | 166 (95.4) | 103 (88.8) | ||
| Sites of metastasis at baseline, n (%) | ||||||
| Bone | 61 (48.4) | – | – | 46 (39.7) | ||
| Brain | 26 (20.6) | 58 (33.9) | 60 (34.5) | 24 (20.7) | ||
| Liver | 26 (20.6) | 30 (17.5) | 35 (20.1) | 19 (16.4) | ||
| Adrenal glands | – | – | – | 22 (19) | ||
| ECOG performance status at screening, n (%) | ||||||
| 0 | 38 (30.2) | 59 (34.5) | 59 (33.9) | 18 (15.5) | ||
| 1 | 88 (69.8) | 112 (65.5) | 115 (66.1) | 97 (83.6) | ||
| Unknown | 0 | – | – | 1 (0.9) | ||
| Previous lines of therapy, n (%) | ||||||
| 0 | 0 | 0 | 0 | 0 | ||
| 1 | 54 (43) | 77 (45.0) | 78 (44.8) | 50 (43.1) | ||
| 2 | 44 (35) | 65 (38.0) | 69 (39.7) | 40 (34.5) | ||
| ≥3 | 28 (22) | 29 (17.0) | 27 (15.5) | 26 (22.4) | ||
| Administered dose | 960 mg every 24 hours | 960 mg every 24 hours | 75 mg/m2 every 3 weeks | 600 mg every 12 hours | ||
| Objective response (95% CI), % | 37.1 (28.6–46.2) | 28.1 | 13.2 | 42.9 (33.5–52.6) | ||
| Best response obtained with the administered treatment, n (%) | ||||||
| Complete | 4 (3.2) | – | – | 1 (0.9) | ||
| Partial | 42 (33.9) | – | – | 47 (42) | ||
| Stable | 54 (43.5) | – | – | 41 (36.6) | ||
| Progression | 20 (16.1) | – | – | 6 (5.4) | ||
| Unknown/not collected/not evaluable | 4 (3.2) | – | – | 15 (15.2) | ||
| Median duration of treatment (range), months | 5.5 (0.2–26.9) | 5.0 (0.1–25.3) | 3.0 (0.75–25.25) | 5.7 (0.03–19.6) | ||
| Treatment related adverse event, % | ||||||
| Any grade | 69.8 | 70 | 86 | 97.4 | ||
| Grade I–II | 49.2 | 37.2 | 45.6 | 52.6 | ||
| Grade III–IV | 20.6 | 32.5 | 39 | 44.8 | ||
| Grade V | 0.8 | 0.005 | 0.01 | 1.7 | ||
| Most frequent adverse events, % | Diarrhea: 31.7 | Diarrhea: 34 | Fatigue: 25 | Diarrhea: 62.9 | ||
| Nausea: 19 | Nausea: 14 | Alopecia: 21 | Nausea: 62.1 | |||
| Increase of ALT: 15.1 | Decreased appetite: 11 | Nausea: 20 | Vomiting: 47.4 | |||
| Dose reduction/dose interruption due to adverse events, % | 22.2 | Dose reduction: 15.4 | Dose reduction: 26.5 | Dose reduction: 51.7 | ||
| Dose interruption: 35 | Dose interruption: 15.2 | Dose interruption: 61.2 | ||||
| Discontinuation of treatment due to adverse events, % | 7.1 | 9.5 | 11.3 | 6.9 | ||
| Disease control ratio (95% CI), % | 80.6 (72.6–87.2) | 82.5 (75.9–87.8) | 60.3 (52.7–67.7) | 79.5 (70.8–86.5) | ||
| Median progression-free survival (95% CI), months | 6.8 (5.1–8.2) | 5.6 (4.3–7.8) | 4.5 (3.0–5.7) | 6.5 (4.7–8.4) | ||
| Median overall survival (95% CI), months | 12.5 (10–not evaluable) | 10.6 (8.9–14.0) | 11.3 (9.0–14.9) | 12.6 (9.2–19.2) | ||
NSCLC, non-small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; ALT, alanine transaminase.
A very interesting part of this study was the sub-analysis of response according to KRAS-associated comutations. In those patients with STK11 and KEAP1 comutations the response rate was 23.1% versus 50% in those patients with STK11 mutated and KEAP1 wild-type. Furthermore, only one patient out of seven (14.3%) responded to sotorasib in tumours with STK11 wild-type and KEAP1 mutated (40). This shows the great importance of KRAS mutations in contrast to other mutations such as ALK or ROS1, which are practically exclusive of other target mutations.
Subsequent to this study, data were obtained from the CodeBreak 200 phase 3 clinical trial comparing sotorasib (171 patients) versus docetaxel (174 patients) in patients with advanced or metastatic KRAS G12C-mutated NSCLC previously treated with chemotherapy and PD-1 or PD-L1 inhibitors. In this clinical trial whose primary endpoint was PFS, PFS was 5.6 months for sotorasib versus 4.5 months for docetaxel [hazard ratio (HR) 0.66; 95% CI: 0.51–0.86]. The grade ≥3 adverse event rate was 33% for sotorasib versus 44% for docetaxel. As in the phase 1/2 study, the most frequent adverse events were gastrointestinal and hepatic (41). Based on the pivotal data from the CodeBreak 100 clinical trial, the FDA approved sotorasib for advanced KRAS G12C-mutated NSCLC in May 2021 (42).
Subsequent to sotorasib, another inhibitor was approved by the FDA in December 2022. Adagrasib is a KRAS G12C inhibitor whose efficacy has been tested in NSCLC in the phase 1/2 KRYSTAL-1 clinical trial. A total of 116 patients were evaluated (98.3% had received prior chemotherapy and immunotherapy) with an ORR of 42.9%. The median PFS was 6.5 months (95% CI: 4.7–8.4) and OS was 12.6 months (95% CI: 9.2–19.2). In patients with central nervous system involvement the ORR was 33.3% (43). The rate of grade ≥3 adverse events was 44.8%, mainly at the expense of gastrointestinal toxicity. Serious adverse events included an anaemia rate of 14.7% and a pneumonia rate of 12.1%. The drug is currently pending approval by the European Medicines Agency (EMA) (Figure 3).
Mechanisms of resistance to KRAS G12C-targeted therapies include the appearance of secondary mutations in the oncogene or the development of secondary pathways in the signalling cascade. In patients with mutated KRAS receiving sotorasib, increased phosphorylation of ERK has been detected, so RAF or MEK pathways may be involved. The EGFR growth receptor may also be involved in the poor efficacy in some patients with KRAS G12 mutations receiving targeted therapy (14). In some models it has been shown that there may be increased activation of EGFR receptor tyrosine kinase activity in patients with mutated KRAS compared to non-mutated KRAS, which is why trials are currently underway proposing the combination of an anti-EGFR with a KRAS inhibitor. Another pathway implicated in resistance is the permanent activation of the PI3K pathway in some patients, which is involved in inhibiting cell death, so the development of specific inhibitors is also being studied (44).
The CodeBreak 300 phase 3 clinical trial evaluated the association of sotorasib with panitumumab (anti-EGFR) in patients with advanced KRAS G12C-mutated colorectal cancer. In this study, the median PFS was 5.6 months for sotorasib-panitumumab (53 patients) versus 2.2 months for standard therapy (54 patients with trifluridine-tipiracil or regorafenib) with an HR of 0.49 (95% CI: 0.30–0.80). The ORR was 26.4% versus 0% respectively (45). The rate of grade ≥3 adverse events was higher in the standard arm with a rate in this case of 43.1% versus 35.8% for the sotorasib-panitumumumab combination (46). Therefore, this study paves the way for reversing KRAS inhibitor resistance in NSCLC in the future.
The appearance of mutations in the KRAS oncogene itself is another of the defined mechanisms of resistance to treatment. Among the secondary mutations identified, some such as G12D, R68M, A59S and A59T show resistance to sotorasib, but are sensitive to treatment with adagrasib. In contrast, the secondary mutation Q61L shows the opposite behaviour. It is feasible to think that the combination of inhibitors against KRAS G12C could significantly improve its efficacy in the future (47). Currently, RAS pan-inhibitors are being developed that can treat all NSCLC-associated mutations, as well as having a more favorable resistance treatment profile than current inhibitors. For example, the molecule BI 1701963 appears to be an oral inhibitor that has been shown to be effective in preclinical models. All treatments developed against RAS in both preclinical and clinical models will be of great importance in the use of immunotherapy both upstream and downstream.
Response to immunotherapy and KRAS mutations
Immunotherapy is currently the main treatment for NSCLC at all stages of the disease. The use of therapies based on anti-PD-1, anti-PD-L1 and anti-CTLA-4 has allowed changing the natural history of NSCLC, with response rates in advanced stages that can achieve up to 20–30% of long survivors. The use of immunotherapy is currently not well known in NSCLC with target mutations. In tumors with EGFR mutations resistance to immune therapies is known, however, in tumors with RAS mutations this is unknown and is under study.
In patients with NSCLC and mutated KRAS G12C, a higher tumor mutational burden (TMB) and high PD-L1 expression have been observed in relation to other mutations in the oncogene. Within this subgroup, some studies have suggested that PD-L1 expression is higher in patients with a high smoking history than in those who never smoked. Initial studies suggested that, although several clinical trials such as KEYNOTE 001 had concluded that high PD-L1 expression in NSCLC should be expected to have a better prognosis with immunotherapy (48), if a KRAS G12C mutation was found, high PD-L1 expression correlated with a worse prognosis in this subgroup. However, the occurrence of comutations next to KRAS such as STK11 or TP53, which in subsequent work have shown a worse prognosis when analysed independently, was not analysed.
Sotorasib has been shown to increase the expression of inflammatory cytokines favouring T-cell infiltration of the tumour area. This suggests that there may be a synergy resulting from the combination of specific KRAS inhibitors and immunotherapy with PD-1 or PD-L1 agents. Some studies such as CodeBreak 100/101 that evaluated the administration of sotorasib in combination with the antibodies atezolizumab or pembrolizumab in NSCLC have shown promising results. Some mutations such as KRAS G12C may be associated with a good response to immunotherapy in combination with specific inhibitors, however, there are discordant results between studies and further validation of this theory and further analysis of other variables that may be involved will be needed in the future (49). In patients with KRAS G12C mutations, increased activation of NF-κB, ERK and PI3K/AKT pathways was observed, which could influence OS. Further work looking at the combination of targeted therapies may shed light on how best to approach these patients in clinical practice.
Mutations other than KRAS G12C appear less frequently in NSCLC, some such as G12V or G13C have been associated, like G12C, with high PD-L1 expression (50). Work in animal models has shown that the G12V mutation is associated with increased PD-L1 expression by promoting lymphocyte migration through the epithelial-mesenchymal transition process (51). On the other hand, this mutation promotes immune evasion through transforming growth factor ß. However, other mutations such as G12A or G12D have been associated with low expression. In the case of the G12D mutation, there are contradictory studies, although an important study presented by Liu et al. suggested that in patients with NSCLC with this mutation there was a lower mutational load and less lymphocyte infiltration, which was related to a worse response to immunotherapy, especially if a TP53 comutation was associated (52). However, given the lower prevalence and the discrete number of studies focused on analysing KRAS mutations other than G12C in NSCLC, it is not possible to draw solid conclusions at present about the influence of immunotherapy in these subgroups.
Conclusions
The KRAS gene has fundamental implications in NSCLC, both at the molecular and clinical levels. The correct and thorough characterisation of the different alterations and mutations of the KRAS gene will open up new avenues in the treatment of KRAS-mutated NSCLC, as well as personalising immunotherapy treatments in patients with these alterations. One of the future of the treatment of KRAS-mutated NSCLC will be the development of RAS pan-inhibitors or the use of combination therapies with immunotherapy or other target drugs that will allow an increase in the survival and response of patients with NSCLC. It is essential to develop new clinical trials that will allow the emergence of new anti-KRAS therapies in NSCLC.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-26/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-26/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-26/coif). A.O.H. serves as an unpaid editorial board member of Precision Cancer Medicine from August 2023 to July 2025. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mithoowani H, Febbraro M. Non-Small-Cell Lung Cancer in 2022: A Review for General Practitioners in Oncology. Curr Oncol 2022;29:1828-39. [Crossref] [PubMed]
- de Castro Carpeño J, Belda-Iniesta C. KRAS mutant NSCLC, a new opportunity for the synthetic lethality therapeutic approach. Transl Lung Cancer Res 2013;2:142-51. [PubMed]
- Zhang Z, Wang Z. K-ras role in lung cancer therapy. Minerva Chir 2011;66:251-68. [PubMed]
- Ulivi P, Zoli W, Chiadini E, et al. EGFR and K-ras mutations in cytologic samples from fine-needle aspirates in NSCLC patients. Eur Respir J 2012;40:267-9. [Crossref] [PubMed]
- Soung YH, Lee JW, Kim SY, et al. Mutational analysis of EGFR and K-RAS genes in lung adenocarcinomas. Virchows Arch 2005;446:483-8. [Crossref] [PubMed]
- Scheffler M, Ihle MA, Hein R, et al. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J Thorac Oncol 2019;14:606-16. [Crossref] [PubMed]
- Selvaggi G, Scagliotti GV. Histologic subtype in NSCLC: does it matter? Oncology (Williston Park) 2009;23:1133-40. [PubMed]
- Reck M, Carbone DP, Garassino M, et al. Targeting KRAS in non-small-cell lung cancer: recent progress and new approaches. Ann Oncol 2021;32:1101-10. [Crossref] [PubMed]
- Pennell NA, Arcila ME, Gandara DR, et al. Biomarker Testing for Patients With Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book 2019;39:531-42. [Crossref] [PubMed]
- Noda N, Matsuzoe D, Konno T, et al. K-ras gene mutations in non-small cell lung cancer in Japanese. Oncol Rep 2001;8:889-92. [Crossref] [PubMed]
- Jonna S, Subramaniam DS. Molecular diagnostics and targeted therapies in non-small cell lung cancer (NSCLC): an update. Discov Med 2019;27:167-70. [PubMed]
- Imyanitov EN, Iyevleva AG, Levchenko EV. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit Rev Oncol Hematol 2021;157:103194. [Crossref] [PubMed]
- Russo A, Franchina T, Ricciardi GRR, et al. Third generation EGFR TKIs in EGFR-mutated NSCLC: Where are we now and where are we going. Crit Rev Oncol Hematol 2017;117:38-47. [Crossref] [PubMed]
- Kalikaki A, Koutsopoulos A, Trypaki M, et al. Comparison of EGFR and K-RAS gene status between primary tumours and corresponding metastases in NSCLC. Br J Cancer 2008;99:923-9. [Crossref] [PubMed]
- Amanam I, Mambetsariev I, Gupta R, et al. Role of immunotherapy and co-mutations on KRAS-mutant non-small cell lung cancer survival. J Thorac Dis 2020;12:5086-95. [Crossref] [PubMed]
- Peng L, Guo J, Kong L, et al. Efficacy of immunotherapy in KRAS-mutant advanced NSCLC: A real-world study in a Chinese population. Front Oncol 2022;12:1070761. [Crossref] [PubMed]
- Lauko A, Kotecha R, Barnett A, et al. Impact of KRAS mutation status on the efficacy of immunotherapy in lung cancer brain metastases. Sci Rep 2021;11:18174. [Crossref] [PubMed]
- Justeau G, Huchot E, Simonneau Y, et al. Impact of KRAS G12C mutation in patients with advanced non-squamous non-small cell lung cancer treated with first-line pembrolizumab monotherapy. Lung Cancer 2022;174:45-9. [Crossref] [PubMed]
- Sciortino C, Viglialoro V, Nucci M, et al. Response to immunotherapy in KRAS G12C mutated NSCLC: a single-centre retrospective observational study. Oncotarget 2022;13:686-93. [Crossref] [PubMed]
- Zhou B, Tang C, Li J. k-RAS mutation and resistance to epidermal growth factor receptor-tyrosine kinase inhibitor treatment in patients with nonsmall cell lung cancer. J Cancer Res Ther 2017;13:699-701. [Crossref] [PubMed]
- Ren J, Li G, Ge J, et al. Is K-ras gene mutation a prognostic factor for colorectal cancer: a systematic review and meta-analysis. Dis Colon Rectum 2012;55:913-23. [Crossref] [PubMed]
- Zhang Y, Meng X, Tang H, et al. Design, synthesis, and biological evaluation of novel substituted thiourea derivatives as potential anticancer agents for NSCLC by blocking K-Ras protein-effectors interactions. J Enzyme Inhib Med Chem 2020;35:344-53. [Crossref] [PubMed]
- Ahmad S, Badr B, Khan A, et al. The Role of K-Ras and P53 in Biliary Tract Carcinoma. J Pak Med Assoc 2021;71:2378-84. [Crossref] [PubMed]
- Ricciuti B, Alessi JV, Elkrief A, et al. Dissecting the clinicopathologic, genomic, and immunophenotypic correlates of KRAS(G12D)-mutated non-small-cell lung cancer. Ann Oncol 2022;33:1029-40. [Crossref] [PubMed]
- Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol 2008;9:962-72. [Crossref] [PubMed]
- Ye N, Xu Q, Li W, et al. Recent Advances in Developing K-Ras Plasma Membrane Localization Inhibitors. Curr Top Med Chem 2019;19:2114-27. [Crossref] [PubMed]
- Le X, Nilsson M, Goldman J, et al. Dual EGFR-VEGF Pathway Inhibition: A Promising Strategy for Patients With EGFR-Mutant NSCLC. J Thorac Oncol 2021;16:205-15. [Crossref] [PubMed]
- Yan D, Huelse JM, Kireev D, et al. MERTK activation drives osimertinib resistance in EGFR-mutant non-small cell lung cancer. J Clin Invest 2022;132:e150517. [Crossref] [PubMed]
- Aran V, Omerovic J. Current Approaches in NSCLC Targeting K-RAS and EGFR. Int J Mol Sci 2019;20:5701. [Crossref] [PubMed]
- de Mello RA, Marques DS, Medeiros R, et al. Epidermal growth factor receptor and K-Ras in non-small cell lung cancer-molecular pathways involved and targeted therapies. World J Clin Oncol 2011;2:367-76. [Crossref] [PubMed]
- Weiss GJ, Ganeshan B, Miles KA, et al. Noninvasive image texture analysis differentiates K-ras mutation from pan-wildtype NSCLC and is prognostic. PLoS One 2014;9:e100244. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Rizzo S, Raimondi S, de Jong EEC, et al. Genomics of non-small cell lung cancer (NSCLC): Association between CT-based imaging features and EGFR and K-RAS mutations in 122 patients-An external validation. Eur J Radiol 2019;110:148-55. [Crossref] [PubMed]
- Roth JA. Modification of mutant K-ras gene expression in non-small cell lung cancer (NSCLC). Hum Gene Ther 1996;7:875-89. [Crossref] [PubMed]
- Li S, Rosell R, Urban A, et al. K-ras gene point mutation: a stable tumor marker in non-small cell lung carcinoma. Lung Cancer 1994;11:19-27. [Crossref] [PubMed]
- Jin H, Jang Y, Cheng N, et al. Restoration of mutant K-Ras repressed miR-199b inhibits K-Ras mutant non-small cell lung cancer progression. J Exp Clin Cancer Res 2019;38:165. [Crossref] [PubMed]
- Piva S, Ganzinelli M, Garassino MC, et al. Across the universe of K-RAS mutations in non-small-cell-lung cancer. Curr Pharm Des 2014;20:3933-43. [Crossref] [PubMed]
- Dy GK, Govindan R, Velcheti V, et al. Long-Term Outcomes and Molecular Correlates of Sotorasib Efficacy in Patients With Pretreated KRAS G12C-Mutated Non-Small-Cell Lung Cancer: 2-Year Analysis of CodeBreaK 100. J Clin Oncol 2023;41:3311-7. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- de Langen AJ, Johnson ML, Mazieres J, et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRAS(G12C) mutation: a randomised, open-label, phase 3 trial. Lancet 2023;401:733-46. [Crossref] [PubMed]
- Brazel D, Kim J, Ou SI. CodeBreaK 200: Sotorasib (AMG510) Has Broken the KRAS G12C+ NSCLC Enigma Code. Lung Cancer (Auckl) 2023;14:31-9. [Crossref] [PubMed]
- Nakajima EC, Drezner N, Li X, et al. FDA Approval Summary: Sotorasib for KRAS G12C-Mutated Metastatic NSCLC. Clin Cancer Res 2022;28:1482-6. [Crossref] [PubMed]
- Jänne PA, Riely GJ, Gadgeel SM, et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRAS(G12C) Mutation. N Engl J Med 2022;387:120-31. [Crossref] [PubMed]
- Awad MM, Liu S, Rybkin II, et al. Acquired Resistance to KRAS(G12C) Inhibition in Cancer. N Engl J Med 2021;384:2382-93. [Crossref] [PubMed]
- Fakih MG, Salvatore L, Esaki T, et al. Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C. N Engl J Med 2023;389:2125-39. [Crossref] [PubMed]
- Zhang SS, Lee A, Nagasaka M. CodeBreak 200: Sotorasib Has Not Broken the KRAS(G12C) Enigma Code. Lung Cancer (Auckl) 2023;14:27-30. [PubMed]
- Parums DV. Editorial: Recent Approval of Sotorasib as the First Targeted Therapy for KRAS G12C-Mutated Advanced Non-Small Cell Lung Cancer (NSCLC). Med Sci Monit 2022;28:e938746. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non-Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Lee A. Sotorasib: A Review in KRAS G12C Mutation-Positive Non-small Cell Lung Cancer. Target Oncol 2022;17:727-33. [Crossref] [PubMed]
- Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001;15:3243-8. [Crossref] [PubMed]
- Johnson L, Mercer K, Greenbaum D, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001;410:1111-6. [Crossref] [PubMed]
- Liu C, Zheng S, Wang Z, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun (Lond) 2022;42:828-47. [Crossref] [PubMed]
Cite this article as: Olivares-Hernández A, Posado-Domínguez L, Martín-Galache M, Redondo-González JC, Roldán-Ruiz J, Bellido-Hernández L, Fonseca-Sánchez E, Barco-Morillo ED. Current and future perspectives in clinical practice in KRAS-mutated non-small cell lung cancer: a literature review. Precis Cancer Med 2025;8:1.


