
Safe management of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) + panitumumab in a patient with metastatic colorectal cancer undergoing hemodialysis for bilateral nephrectomy: a case report
Highlight box
Key findings
• This is a case of a dialysis patient for previous bilateral nephrectomy with metastatic colorectal cancer safely treated with 5-fluorouracil, leucovorin, oxaliplatin, and panitumumab.
What is known and what is new?
• Patients with end-stage renal disease (ESRD) are frail individuals at risk of complications.
• Treatment of cancer patients on dialysis represents an unsolved problem due to the scarcity of available data and the difficulty of carrying out prospective studies.
• To our knowledge, this is one of the few cases described of chemotherapy in a patient with ESRD for bilateral nephrectomy.
What is the implication, and what should change now?
• Multidisciplinary management of ESRD frail patients can offer a safe oncological cure.
• Hemodialysis patients with ESRD should not be excluded a priori from chemotherapy.
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality worldwide and is associated with high morbidity (1).
Based on tumor molecular characteristics, treatment for metastatic CRC (mCRC) primarily consists of systemic chemotherapy (fluoro-pyrimidines, oxaliplatin and/or irinotecan) in association with monoclonal antibodies targeting the epidermal growth factor receptor (EGFR) or the vascular endothelial growth factor (VEGF) (2). Particularly, in the absence of activating mutations of RAS and BRAF genes, combinations of chemotherapy with anti-EGFR agents, such as cetuximab or panitumumab, are valuable options especially in patients with left-sided colon cancer. Tumors displaying either RAS or BRAF mutations, although, are resistant to anti-EGFR therapies and are commonly treated with combinations of chemotherapy and anti-angiogenic drugs (3).
These cytotoxic drugs, as well as monoclonal antibodies, are quite safe and manageable. However, there are situations in which their use is contraindicated or they should be used with caution. In patients with acute renal failure or severe liver dysfunction, for example, the dose of drugs would be reduced or, as well, some chemotherapeutic agents omitted. In case of hypersensitivity to oxaliplatin, specific desensitization protocols must be followed, while patients harboring mutations inactivating in the dihydropyrimidine dehydrogenase (DPYD) gene, that encodes the enzyme responsible for the metabolism of fluoropyrimidines, are intolerant to standard doses of 5-fluorouracil (5-FU) or capecitabine.
Relatively to those patients dialyzed for end-stage renal disease (ESRD)—or for the rare event they have no kidneys—that need anti-cancer therapies for mCRC, there are many concerns about the safety of chemotherapy, since a paucity of data are actually available.
Here, we report the case of a patient with mCRC on hemodialysis (HD) for previous bilateral nephrectomy who was treated with an association of 5-FU, leucovorin, and oxaliplatin (FOLFOX), and panitumumab. We present this case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-1/rc).
Case presentation
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
In August 2021, a sub-stenosing adenocarcinoma of the left colic flexure was diagnosed by colonoscopy in a 69-year-old Caucasic man after recurrent episodes of constipation. A computed tomography (CT) scan with contrast confirmed the left colon tumor with metastatic loco-regional nodes and showed multiple (>10) bilateral liver metastases. Both carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) levels were increased (22.6 ng/mL and 125 U/mL, respectively).
His medical history included arterial hypertension on treatment, hypothyroidism secondary to thyroidectomy, benign prostatic hypertrophy, cholecystectomy, emergency right nephrectomy in 1968 due to kidney rupture secondary to trauma, left nephrectomy in 1996 due to a renal mass which was histologically negative for neoplasm. Since 1996, the patient undergoes three-weekly HD treatment due to the ESRD secondary to bilateral nephrectomy. The patient reported no smoking or alcohol habit, while illness family history was not relevant.
Considering the risk of bowel obstruction, the patient underwent a palliative left hemicolectomy with terminal colostomy; during surgery, some isolated nodules suspected for carcinosis of the abdominal wall were found and resected. Histological examination revealed a pT4 poorly differentiated adenocarcinoma; the margins of resection were negative; peritoneal nodules as well as 7 of 14 lymph nodes were positive for metastasis of adenocarcinoma (pN2b M1).
The patient was then evaluated by an oncologist from another center but did not receive any indications for antineoplastic systemic treatment due to high risk of toxicity for his ESRD. He finally came to our attention in December 2021 for a second opinion. The patient was in fair general clinical condition; he complained of asthenia and postprandial abdominal pain which subsided with evacuation. The CT scan confirmed multiple bilobar hepatic metastases (max diameter, 3.5 cm) and peritoneal carcinosis. Molecular analysis of KRAS, NRAS, and BRAF genes on tumor sample did not show any pathogenic mutations. Microsatellite analysis resulted stable. The analysis of single nucleotide polymorphisms (SNPs) of the DPYD revealed a heterozygous variant (c.2194G<A) requiring a dose reduction of 25% for the use of fluoropyrimidines. Blood chemists showed hypochromic-microcytic anemia [hemoglobin (Hb), 8.1 g/dL], and a slight increase in gamma-glutamyl transferase (79 IU/L) and phosphatase alanine (207 IU/L); both CEA and CA19-9 levels were incremented, respectively 78.2 ng/mL and 801.6 IU/mL.
Considering the good performance status (PS) and both clinical and molecular characteristics of the tumor (left-sided; RAS/BRAF wild type), in agreement with the reference nephrologist, we decided to start a first-line chemotherapy with FOLFOX-panitumumab [oxaliplatin 85 mg/m2 intravenously (IV), over 90 minutes; folinic acid 400 mg/m2 IV, over 120 minutes; 5-FU 400 mg/m2 IV bolus, then 2,400 mg/m2 IV infusion over 46 hours; panitumumab 5 mg/kg IV infusion over 1 hour; every 2 weeks], continuing the three-weekly HD treatment. Dosage of chemotherapy drugs was globally reduced to 50–60% as a precaution for ESRD and DPYD. The first day of each cycle was always carried out on Monday, while HD was normally performed on Tuesdays, Thursdays, and Saturdays. Close monitoring of blood count, kidney function, and electrolytes was performed before each dialysis session. In addition to comorbid medications, the patient was taking corticosteroids and antiemetics for 48 hours after chemotherapy, granulocyte growth factors (filgrastim) for secondary prophylaxis, renal-dosed alpha erythropoietin for anemia and transdermal fentanyl to control pain.
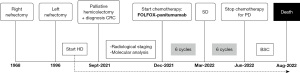
Between December 2021 and June 2022, he completed 12 cycles of FOLFOX-panitumumab without any significant adverse effect, except for acneiform rash (G2) treated as per clinical practice and neutropenic incidents (G2–3) treated with granulocyte colony-stimulating factor (G-CSF). Chemotherapy was administered in reduced doses. Patient underwent two infusions of chemotherapy with doses reduced to 50% for PS and comorbidities; the good tolerance to the treatment allowed us to increase the dosages to 60%: 5-FU in relation to the result of the SNPs (DPYD) and oxaliplatin in relation to the renal function [creatinine clearance (CrCL) <30 mL/min]. After approximately 3 months of chemotherapy (6 cycles), the CT scan described a stable disease according to the related Response Criteria (rRC), although a noticeable improvement of the clinical condition of the patient, in particular the resolution of abdominal pain resulting in a reduction in opioid dosage. The reevaluation by CT performed after 12 cycles of FOLFOX-panitumumab showed disease progression. The patient’s clinical conditions suddenly worsened (inappetence, marked asthenia, and the need for frequent transfusions due to persistence of Hb values <8 g/dL). In consideration of the progression of the disease and the patient’s PS, a new line of active oncological treatment was not considered, favoring palliative care and support at home. Patient died in August 2022. A timeline representation of the patient’s history is depicted in Figure 1.
Discussion
In recent decades, the number of dialysis patients with mCRC has been increased due to the improvement of life expectancy despite renal disease (4). Drugs commonly used in mCRC include various combinations of fluoropyrimidines, oxaliplatin, irinotecan, and monoclonal antibodies against EGFR or VEGF, whose safety and efficacy, however, have been poorly investigated in HD.
HD increases the risk of chemotherapy overdoses or excessive drug clearance with consequent ineffective doses. Based on pioneering studies on in vitro pharmacokinetic of 20 chemotherapeutic agents (5), drugs have been historically classified into “good, intermediate, and ineffective” dialyzable. Several variables, however, are responsible of in vivo pharmacodynamics, including: (I) drug molecular weight; (II) volume of distribution; (III) tissues-to-plasma transfer-drug rate; and (IV) transformation of drugs into active or inactive metabolites (6).
Although there are no real concerns about the use of 5-FU and monoclonal antibodies in patients with ESRD, given their very low renal excretion (7,8), data on the use of irinotecan and oxaliplatin are very limited.
Our experience with a common oxaliplatin-based regimen (FOLFOX) in association with panitumumab in a HD patient for previous bilateral nephrectomy was comparable, in terms of safety and manageability, with respect to the general population treated with the same drugs. This suggests some considerations on the possibility of using oxaliplatin also in patients with ESRD, removing possible fears and perplexities.
Oxaliplatin is an alkylating agent that blocks DNA transcription and replication. About 73% of infused oxaliplatinum rapidly binds to plasma proteins and erythrocytes or is eliminated through the urine, while the platinum remaining in the systemic circulation (free platinum) is responsible for anticancer activity and side effects (9). Of note, after oxaliplatin infusion, most of the circulating platinum in plasma consists of inactive biotransformation products. One hour after the end of the infusion, most of the unbound platinum consists of inactive low molecular weight platinum conjugated with glutathione, L-methionine, and L-cysteine, while only ≤12% of the unbound platinum remains unchanged. After 3 hours, free oxaliplatin constitutes <5% of the unbound circulating platinum (10). These observations are consistent with the hypothesis that the active form of oxaliplatin is cleared within the first hours after infusion by mechanisms independent from renal function. Subsequently, biologically inactive low molecular weight platinum is excreted via glomerular filtration, which could explain why renal failure does not increase oxaliplatin-related toxicity (11).
Generally, the use of oxaliplatin is discouraged in patients with CrCL <30 mL/min. Although no specific dosage has been established in HD patients with ESRD, previous reports would suggest that toxicity do not increase for patients with impaired renal function (12-15). Moreover, a study by Watayo et al. investigated the use of oxaliplatin in dialysis and showed that free platinum is completely eliminated by HD (16).
Typical concerns when planning chemotherapy in a dialysis patient is dosage choice and timing of drug administration in relation to HD sessions. Dose reductions are often required to optimize drug exposure, ensure efficacy, and reduce the risk of side effects (12). Relatively to oxaliplatin, however, there are only few data on systemic treatment modalities in dialysis patients, particularly regarding dose adjustment and timing of HD. Gori et al. proposed a 50% dose reduction of oxaliplatin for dialysis patients with mCRC, maintaining the standard biweekly schedule and performing HD on the same day of drug infusion (17). Anyway, oxaliplatin dose reduction may not be necessary if HD is performed soon after the infusion, as suggested by a study monitoring plasma concentration of total and free platinum in patients undergoing dialysis (16).
Overall, the absence of satisfactory case series, the lack of randomized clinical trials, and the absence of reliable data on the efficacy and safety of standard chemotherapy regimens in patients with ESRD often leads clinicians to consider only best supportive care for dialysis patients. In this context, we decided to treat with FOLFOX and panitumumab a patient with mCRC and ESRD requiring three-weekly dialysis. Notably, preliminary investigations revealed a SNP of DPYD, which affect enzymatic activity and the severity of fluoropyrimidine toxicity. Our experience can be considered positive as the patient stopped treatment after about 6 months due to progressive disease, without having experienced serious adverse events related to chemotherapy. Moreover, during the treatment period we observed clinical improvement for symptom relief and a relative stability of the disease. To obtain these results, the following precautions have been implemented: (I) the infused liquids have been reduced to a minimum respecting the dilutions that guarantee pharmacological stability; (II) the dialysis sessions were performed within 24 hours immediately following chemotherapy infusion (day 1) and at the end of 5-FU continuous infusion (day 3).
Conclusions
The treatment of dialysis cancer patients represents an unsolved issue due to the scarcity of data available and the difficulty of carrying out prospective studies. Furthermore, patients with ESRD are frail subjects at risk of complications that, in the event of a diagnosis of cancer, could receive treatments potentially incompatible with their kidney disease or capable of abruptly altering their delicate clinical balance. We reported a case of a dialysis patient for previous bilateral nephrectomy with mCRC and SNP of DPYD safely treated with FOLFOX and panitumumab. Our experience strengthens that dialysis is not an absolute contraindication to systemic treatments, as long as multidisciplinary management is always guaranteed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-1/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-23-1/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Guo TA, Wu YC, Tan C, et al. Clinicopathologic features and prognostic value of KRAS, NRAS and BRAF mutations and DNA mismatch repair status: A single-center retrospective study of 1,834 Chinese patients with Stage I-IV colorectal cancer. Int J Cancer 2019;145:1625-34. [Crossref] [PubMed]
- Raimondi A, Fucà G, Leone AG, et al. Impact of age and gender on the efficacy and safety of upfront therapy with panitumumab plus FOLFOX followed by panitumumab-based maintenance: a pre-specified subgroup analysis of the Valentino study. ESMO Open 2021;6:100246. [Crossref] [PubMed]
- Maruta M, Miyoshi T, Matsuo N, et al. Clinical pharmacokinetics of oxaliplatin in a hemodialysis patient with advanced gastric cancer. J Chemother 2021;33:51-5. [Crossref] [PubMed]
- Sauer H, Füger K, Blumenstein M. Modulation of cytotoxicity of cytostatic drugs by hemodialysis in vitro and in vivo. Cancer Treat Rev 1990;17:293-300. [Crossref] [PubMed]
- Pedrazzoli P, Silvestris N, Santoro A, et al. Management of patients with end-stage renal disease undergoing chemotherapy: recommendations of the Associazione Italiana di Oncologia Medica (AIOM) and the Società Italiana di Nefrologia (SIN). ESMO Open 2017;2:e000167. [Crossref] [PubMed]
- Kobuchi S, Ito Y. Application of Pharmacometrics of 5-Fluorouracil to Personalized Medicine: A Tool for Predicting Pharmacokinetic-Pharmacodynamic/Toxicodynamic Responses. Anticancer Res 2020;40:6585-97. [Crossref] [PubMed]
- Lo L, Patel D, Townsend AR, et al. Pharmacokinetic and pharmacodynamic evaluation of panitumumab in the treatment of colorectal cancer. Expert Opin Drug Metab Toxicol 2015;11:1907-24. [Crossref] [PubMed]
- Wang D, Li X, Xu L, et al. Dose-escalation of oxaliplatin in hemodialysis patient treated with FOLFOX therapy: A case report. Medicine (Baltimore) 2019;98:e17462. [Crossref] [PubMed]
- Allain P, Heudi O, Cailleux A, et al. Early biotransformations of oxaliplatin after its intravenous administration to cancer patients. Drug Metab Dispos 2000;28:1379-84. [PubMed]
- Takimoto CH, Graham MA, Lockwood G, et al. Oxaliplatin pharmacokinetics and pharmacodynamics in adult cancer patients with impaired renal function. Clin Cancer Res 2007;13:4832-9. [Crossref] [PubMed]
- Janus N, Thariat J, Boulanger H, et al. Proposal for dosage adjustment and timing of chemotherapy in hemodialyzed patients. Ann Oncol 2010;21:1395-403. [Crossref] [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [Crossref] [PubMed]
- Takimoto CH, Remick SC, Sharma S, et al. Dose-escalating and pharmacological study of oxaliplatin in adult cancer patients with impaired renal function: a National Cancer Institute Organ Dysfunction Working Group Study. J Clin Oncol 2003;21:2664-72. [Crossref] [PubMed]
- Massari C, Brienza S, Rotarski M, et al. Pharmacokinetics of oxaliplatin in patients with normal versus impaired renal function. Cancer Chemother Pharmacol 2000;45:157-64. [Crossref] [PubMed]
- Watayo Y, Kuramochi H, Hayashi K, et al. Drug monitoring during FOLFOX6 therapy in a rectal cancer patient on chronic hemodialysis. Jpn J Clin Oncol 2010;40:360-4. [Crossref] [PubMed]
- Gori S, Lunardi G, Inno A, et al. Pharmacokinetics of oxaliplatin in a hemodialyzed patient: chemotherapy dose adjustment and timing of dialysis. Clin Colorectal Cancer 2014;13:260-3. [Crossref] [PubMed]
Cite this article as: Filoni E, Musci V, Dibisceglia G, Mannavola F. Safe management of 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) + panitumumab in a patient with metastatic colorectal cancer undergoing hemodialysis for bilateral nephrectomy: a case report. Precis Cancer Med 2024;7:4.

