
Pathologically documented brain necrosis: response to bevacizumab after irradiation for solitary fibrous tumour/haemangiopericytoma: case report and literature review
Highlight box
Key findings
• First successful treatment of cerebral radionecrosis in solitary fibrous tumours/haemangiopericytomas (SFT/HPC) with bevacizumab
What is known and what is new?
• Bevacizumab has an emerging role in the treatment of cerebral radionecrosis.
• To our knowledge, this is the first reported case of radionecrosis post radiotherapy for SFT/HPC, and its successful treatment with bevacizumab.
What is the implication, and what should change now?
• Bevacizumab should be considered early in the course of cerebral radionecrosis.
Introduction
Background
Cerebral radionecrosis occurs in up to 10–15% of cases where cerebral tumours are irradiated with curative intent (1). Its diagnosis is sometimes challenging, with differential diagnoses including tumour pseudoprogression and tumour progression. Management possibilities for radionecrosis are limited, but have primarily included steroids, with their wide-ranging long-term complications, and other therapies which are not often employed. More recently, bevacizumab has been used for radionecrosis (2). Here, we report a case of cerebral radionecrosis that responded to bevacizumab. To our knowledge, this is the first reported case of radionecrosis post radiotherapy for solitary fibrous tumours/haemangiopericytomas (SFT/HPCs), and its successful treatment with bevicizumab.
SFT/HPCs are uncommon vascular mesenchymal tumours which, when they occur intracranially, can mimic angiomatous meningiomas. The incidence of SFT/HPC is around 0.06 per 100,000 individuals (3). They occur mainly in middle-aged individuals and with a similar sex distribution. To date, there are not clear guidelines for management of these tumours. Treatment usually involves surgical resection with or without preoperative embolization, followed by high dose radiotherapy. These tumours are prone to local, distant intracerebral and extracranial recurrences, most commonly to bone, lung and liver (4). As there are no guidelines for post treatment surveillance for patients with these tumours, most relapses present with symptomatic metastases (4).
Although palliative chemotherapy shows modest antitumour efficacy (3), evidence suggests that targeted antiangiogenic agents, addressing the predominantly angiogenic hallmark of these cancers, are becoming rapidly the new standard of care in the setting of metastatic disease.
Rationale and knowledge gap
Bevacizumab has an emerging role in the treatment of cerebral radionecrosis (2,5). Its efficacy is being established for cerebral radiation necrosis in general.
Objective
To our knowledge, this case report is unique in that it is the first reported case of radionecrosis post radiotherapy for SFT/HPC, and its successful treatment with bevacizumab. We present this case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-47/rc).
Case presentation
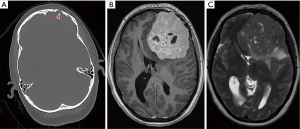
A 36-year-old female was diagnosed with a left frontal SFT/HPC. She complained of headaches and had been noted to have had personality changes over the preceding 8 weeks. Cranial computed tomography showed a 75mm maximal diameter left frontal tumour, confirmed on magnetic resonance imaging (MRI) (Figure 1), with focal skull invasion. In October 2019, she underwent a stereotactic craniotomy and excision of the left frontal brain lesion. The procedure was complicated by massive blood loss.
Histopathology from her initial craniotomy showed a mesenchymal tumour with a bland fibroblastic appearance, with many individual cells surrounded by a small amount of collagen or reticulin. Focally there were prominent dilated blood vessels and scattered regions of necrosis. Mitotic activity was apparent in many areas, but not >10 per high-powered field. There were no psammoma bodies or whorling configurations. The tumour was adjacent to dura mater and there was focal evidence of possible brain invasion. Excision appeared complete. The tumour showed strong diffuse expression of vimentin and STAT6, with focal expression of CD34, EMA and progesterone receptor. The findings, including the immunophenotype, were in keeping with SFT/HPC, World Health Organization (WHO) Grade 2.
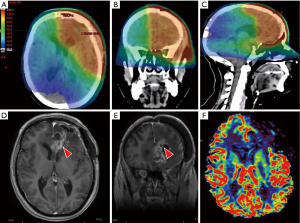
Due to the high risk of locoregional recurrence with SFT/HPC, the patient underwent postoperative radiotherapy to the tumour bed: 60 Gy in 30 fractions using volumetric modulated arc therapy (VMAT) (Figure 2A-2C), which was completed in January 2020.
One month later, she developed a subdural empyema at the operative site, which responded to craniectomy, washout and intravenous antibiotics. She completed the latter in mid-April 2020.
Approximately 6 months post radiotherapy, the patient developed symptomatic brain necrosis, with right facial droop, mild expressive dysphasia, and difficulty in concentration. Visual field (VF) assessment showed bilateral VF deficits (left more than right) with pallor of both optic nerves. Serial MRI scans showed (I) progression of enhancing margins along the medial frontal parafalcine resection cavity, with a solid component, crossing the corpus callosum and (II) a periventricular enhancing lesion around the frontal horn of the left lateral ventricle, with surrounding vasogenic oedema (Figure 2D,2E). It was unclear whether the radiological changes represented recurrent SFT/HPC, pseudo-progression or radiation necrosis. In June 2020, she underwent repeat craniotomy and biopsy of part of the MRI-enhancing solid region adjacent to the surgical cavity. Histopathological features included reactive gliosis, hypercellularity, chronic inflammation and neuronal cell loss, with vascular hyalinisation and reactive endothelial atypia, and other features in keeping with post-radiation necrosis. There was no evidence of SFT/HPC.
A timeline for the patient’s clinical course and radiology is presented in Figure 3.
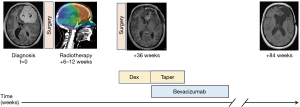
The Neuro-oncology Multidisciplinary Team recommended treatment with 4 cycles of bevacizumab (Avastin), which, at the time of the recommendation, was not subsidized for this indication in the Australian Pharmaceutical benefits Scheme (PBS). With a diagnosis of post-radiation cerebral necrosis, the patient was commenced on treatment with dexamethasone at the dose of 4 mg/day, pending approval of application for compassionate access to bevacizumab. The patient was on dexamethasone for 16 weeks; the bevacizumab overlapped with dexamethasone for 8 weeks (it took approx. 8 weeks until bevacizumab became available). The neurological exam prior initiating dexamethasone was unremarkable, except for mild right facial droop. There were no other cranial nerve abnormalities. Subjectively, the patient complained of difficulties in concentration, but examination of higher centres was unremarkable. The Mini Mental State Examination was also normal. Tone, power, reflexes, coordination and sensation were unremarkable. Unfortunately, there was no MRI in March 2021 prior initiation of either steroids or later, to bevicuzumab.
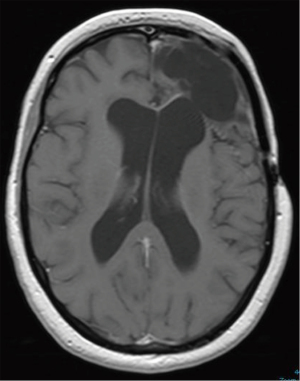
The symptoms (mild right facial droop, morning headaches and difficulties with concentration) responded promptly to dexamethasone 4 mg/day. When the application for compassionate access was approved, treatment with bevacizumab was initiated. This allowed the dexamethasone to be tapered to 0.5 mg/day (halving the dose every 3 days), without any relapse of the symptoms on decreasing the dose. Eventually, the dexamethasone was ceased, 4 cycles after bevacizumab (8 weeks) was initiated. The further 6 cycles of bevacizumab were then delivered in the absence of steroids (Figure 3). Although the Neuro-oncology Multidisciplinary Team recommended 4 cycles of bevacizumab, as the drug subsequently became widely available as a biosimilar, and the patient tolerated the treatment and continued to improve, she received bevacizumab for another 6 cycles. Not only was her dexamethasone able to be tapered without symptoms reoccurring, but her most recent MRI brain (2/8/2021) showed a dramatic improvement compared with April 2020. There was no residual enhancement at the resection site and no residual SFT/HPC was conspicuous (Figure 4). Interval MRI brain on 6/12/2021 and on 11/3/2022 documented stable appearances. However, there was some difficulty in attributing with certainty the clinical and radiological improvement fully to bevacizumab, since the patient had responded to steroids and there was a period during which the two drugs were being delivered simultaneously. Yet the overall picture favoured a bevacizumab response.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Key findings
Cerebral radionecrosis occurs in 10–15% of patients with intracranial tumours, post treatment with conventional or stereotactic radiotherapy (1,6,7). It typically occurs from 3 months to up to three years after irradiation, although later cases have been reported (1,7).
Risk factors for cerebral radionecrosis include total dose, dose per fraction, treatment duration, volume of brain irradiated, concurrent chemotherapy, previous radiotherapy and male sex (1,7). Different brain regions may have different susceptibilities to radionecrosis; symptoms are dependent upon location of radionecrosis, with either focal deficits or more global evidence of increased intracranial pressure. Seizures can occur (1).
The pathophysiology of radionecrosis is not yet completely understood (7). It is hypothesized that high dose focused radiotherapy produces endothelial injury, which initiates the process of necrosis. Studies of histological samples of individuals with brain radionecrosis consistently found vascular changes, oedema, fibrinoid deposition and inflammatory infiltrates (8), as in our case. It is hypothesized that endothelial injury causes apoptosis of endothelial cells and triggers release of inflammatory mediators, chemotactic factors and overexpression of vascular endothelial growth factor (VEGF) (6). In addition, endothelial cell necrosis compromises the integrity of the blood brain barrier, increases vasogenic oedema, promotes release of pro-inflammatory agents, generates thrombi of platelets and fibrin, initiates proliferation of smooth muscle and accumulation of fibroblasts. Overall, these processes result in vascular occlusion, fibrinoid necrosis of the vascular walls, and vasogenic oedema (6,8). The vasogenic oedema leads to ischemia, which generates hypoxia. The latter initiates a cascade of production of hypoxia-inducible factor 1α (HIF1α), which, in turn, leads to overexpression of VEGF in peri-necrotic white matter (8,9). Pre-clinical data have shown upregulation of VEGF in astrocytes and endothelial cells surrounding the area of necrosis (6,8). VEGF increases vascular permeability, further aggravating the oedema (9).
The radiological appearance of radionecrosis is very similar to that of tumour progression and pseudo-progression. Surgical biopsy represents the gold standard to differentiate between these entities. However, the MRI apparent diffusion coefficient (ADC) and relative cerebral blood volume (rCBV) may assist with non-invasive differential diagnosis. The ADC is decreased in tumour progression, due to the high number of cells, limiting movement of water, as opposed to radionecrosis (10). Perfusion MRI applies contrast enhancement to assess the rCBV and analyse vascularization and blood flow. Thus, increased perfusion suggests tumour recurrence (rCBV >2.6) and decreased perfusion, as observed in our case (Figure 2F), strongly suggests radionecrosis (rCBV <0.6) (11).
Characteristic spatiotemporal patters of radionecrosis are described, with a predilection for periventricular locations (12). Such a periventricular location was observed in our case (see Figure 2D,2E).
Until recently, the main therapy for radionecrosis has been corticosteroids, which are able to repress the inflammatory response underlying radionecrosis and achieve relief of symptoms, by decreasing vasogenic oedema (5). However, steroid use in the long term has well-known significant side effects and is not recommended. Another intervention for radionecrosis is surgery in selected cases (7). However, due to its invasive nature and related morbidity, it is not widely used. Hyperbaric oxygen in the treatment of radiation induced brain injury is underpinned by physiological changes induced by high oxygen concentrations, such as augmented angiogenesis and increased blood flow to injured areas, leading to accelerated healing (13), although it is not a widely used radionecrosis therapy.
Ours is an anecdotal case of successful radionecrosis treatment with bevacizumab over five months. There have been two controlled randomised trials of bevacizumab for the treatment of central nervous system (CNS) radiation necrosis. The first was after irradiation of head and neck or brain tumours, but the numbers were very small: n=14 (14). However, all 7 of the bevacizumab-treated patients responded in terms of improvements in CNS symptoms or signs, whereas none in the placebo group did. These investigators suggested that bevacizumab now has an established role for the treatment of cerebral radiation necrosis. In the second trial, bevacizumab responses were compared with responses to corticosteroids in 112 nasopharyngeal cancer patients treated with radiotherapy; patients in the bevacizumab arm showed enhanced symptomatic and radiological responses (15).
We believe that ours is likely a case of successful treatment of brain radiation necrosis with bevacizumab over a 5-month period. However, as for any brain resection/biopsy of suspected radiation necrosis, we cannot exclude that the post-treatment biopsy was a false negative due to sampling error, or that the patient had at least a component of SFT/HPC recurrence, or that it represented pseudo-progression. The time-frame for occurrence was compatible with the latter, which is more commonly seen after radiotherapy for the treatment of high-grade glioma, most usually in conjunction with temozolomide chemotherapy. We cannot exclude that this was the situation for our patient. There is evidence suggesting that radionecrosis may occur as early 3 months post radiotherapy (16). In addition, the histology of the biopsy of the part of the MRI-enhancing solid region adjacent to the surgical cavity displayed hallmarks of radionecrosis, including vascular hyalinisation and reactive endothelial atypia, ischaemic injury and necrotic changes with foamy histiocytes. There are also reports of vascular SFT/HPC tumours responding to regimens including bevacizumab (17) but we consider this unlikely in our case given the absence of symptoms or imaging findings of relapse three months after finishing bevacizumab.
SFT/HPCs are uncommon vascular mesenchymal tumours, which may occur at any site in the body; they can occur intracranially (0.4% of intracranial tumours and less than 2.4% of meningeal neoplasms) (18). Almost two thirds (62%) of intracranial SFT/HPC are supratentorial. The pathological entity SFT/HPC was established in the 2016 WHO classification of CNS tumours (19). The molecular hallmark of these neoplasms is the gene fusion NAB2-STAT6 on chromosome 12 (20). The chimaeric protein product of this gene fusion has the early growth response (EGR) binding domain of NAB2 (a transcriptional inhibitor of factors promoting differentiation and proliferation) and the transactivation domain of STAT6 (a transcription factor involved in cytokine signalling) (20). As a result, this abnormal protein induces EGR signalling, which drives tumorigenesis in SFT/HPC (20).
According to the pathological features of SFT/HPC, such as cellularity, mitotic index, necrosis and nuclear atypia, the 2016 WHO Classification of CNS tumour distinguishes WHO grade I, grade II or grade III tumours (19). While SFT/HPC grade I are considered benign tumours, grade II and grade III lesions are malignant.
To date, the standard of care for SFT/HPC has been surgical resection (with or without preoperative embolization) followed by adjuvant radiotherapy (18). Gross total resection results in superior outcomes compared to subtotal resection (21). Grade II and grade III SFT/HPC are prone to relapse, either intracranially or extracranially (22). Therefore, in patients with grade II and grade III SFT/HPC, surgical resection is followed by adjuvant radiotherapy (18,20). Adjuvant radiotherapy yields considerably superior local control (22) and progression free survival (22) to surgery alone and, arguably, provides a survival benefit (23). However, these tumours carry a significant risk of extracranial metastasis, with rates ranging between 2.6% and 56% (4). As there are no clear guidelines for monitoring patients with these tumours post-surgical treatment + adjuvant radiotherapy, most relapses present with symptomatic metastases (4). Salvage chemotherapy provides a modest benefit in patients with recurrent SFT/HPC (24).
The finding that STAT6 activation is involved in angiogenic signalling (25) opens an avenue for novel therapies for SFT/HPC, such as antiangiogenic agents (26). In addition, the NAB2-STAT6 fusion protein induces carcinogenesis in SFT/HPC by transforming the EGR inhibitor into an EGR activator, with activation of downstream signalling of the EGR pathway via fibroblast growth factor receptor 1 (FGFR1), neurotrophic receptor tyrosine kinase 1 (NTRK1) and insulin-like growth factor 2 (IGF2) providing a biological rationale for potential new therapies for SFT/HPC (20). Agents acting on EGR downstream targets, such as VEGF-A (bevacizumab-used in combination with temozolomide), PDGFRβ and VEGFR1-3 (sunitinib, sorafenib) or PDGFR and VEGFR1-3 (pazopanib) have shown activity in SFT/HPC (27).
Strengths and limitations of this study
Strengths include the well-documented clinical and radiological features of this case, the fact that it appears to be the first case of SFT/HPC reported who suffered cerebral radionecrosis, and that the cerebral necrosis was successfully treated with bevacizumab. Further, we provide substantial literature background on SFT/HPC and cerebral radionecrosis. Limitations of our study include the lack of serial MRIs on this case, especially around the time of bevacizumab use.
Comparison with similar research
Gonzales et al. [2007] (2) first demonstrated the efficacy of bevacizumab in the treatment of CNS radiation necrosis. VEGF signalling boosts permeability of blood vessels in the injured area, impairs the blood-brain barrier (BBB) and induces oedema of the brain (28). By inhibiting the VEGF pathway, bevacizumab decreases vascular permeability, restores the integrity of the BBB and reduces brain oedema (28,29). The crucial role of VEGF signalling in the development of radionecrosis provides a strong biological rationale for using bevacizumab for its treatment (7). There is increasing evidence that bevacizumab is beneficial in the treatment of cerebral radiation necrosis (5,18,28,30), but the length of time that bevacizumab therapy should be maintained in the treatment of cerebral radiation necrosis is yet to be defined. Available data indicate a mean duration of treatment with bevacizumab of around 3 months is optimal (5). It was suggested that, since the aim of treatment is symptomatic relief, therapy should continue until symptoms are relieved, but not necessarily until imaging improves (28). For patients with a recurrence of symptoms, bevacizumab should be reinitiated. On the other hand, recurrence of brain necrosis was reported after cessation of bevacizumab (3,14,31). There is some evidence that prolonged bevacizumab use is counter-productive: first, bevacizumab resistance can develop after long-term use (32,33) and second, protracted use may lead to an exacerbation of necrosis itself, hypothesized to act through bevacizumab-associated vessel over pruning, thereby exacerbating ischaemia and hypoxia in the necrotic area (28). Hence, it seems prudent to use bevacizumab only for as long as necessary to relieve symptoms, but to reinitiate it in cases of symptomatic recurrence. Asymptomatic cases should probably be monitored.
Explanation of findings
Is provided in the previous section.
Implications and actions needed
It may be useful to consider a prospective trial to ascertain the optimal duration of bevacizumab treatment, as the biosimilar bevacizumab is now widely available on the PBS in Australia. In addition, more work needs to be done in prevention of radionecrosis. Radiomics in the era of artificial intelligence and a registry of Medicare data could be a way ahead. Similarly, improving the diagnostic criteria for differentiation between tumour recurrence, pseudprogression and radionecrosis remains an area of significant unmet need.
Conclusions
To our knowledge, this is the first reported case of radionecrosis post radiotherapy for SFT/HPC, and its successful treatment with bevacizumab. Interestingly, this is a unique situation when the angiogenic pathway underpins both the brain neoplasia and the pathophysiology of radionecrosis. In brain radionecrosis, treatment with bevacizumab appears safe and effective. Our patient showed clinical and radiological improvement after completing five months of treatment with bevacizumab and has no evidence of radionecrosis at three months post completion of treatment.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-47/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-47/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leibel S, Sheline G. Tolerance of the brain and spinal cord to conventional therapeutic irradiation. In: Gutin P, Leibel S, Sheline G. editors. Radiation Injury to the Nervous System. New York, NY: Raven Press; 1991:239.
- Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys 2007;67:323-6. [Crossref] [PubMed]
- Wang Y, Pan L, Sheng X, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur J Med Res 2012;17:25. [Crossref] [PubMed]
- Ratneswaren T, Hogg FRA, Gallagher MJ, et al. Surveillance for metastatic hemangiopericytoma-solitary fibrous tumors-systematic literature review on incidence, predictors and diagnosis of extra-cranial disease. J Neurooncol 2018;138:447-67. [Crossref] [PubMed]
- Khan M, Zhao Z, Arooj S, et al. Bevacizumab for radiation necrosis following radiotherapy of brain metastatic disease: a systematic review & meta-analysis. BMC Cancer 2021;21:167. [Crossref] [PubMed]
- Rahmathulla G, Marko NF, Weil RJ. Cerebral radiation necrosis: a review of the pathobiology, diagnosis and management considerations. J Clin Neurosci 2013;20:485-502. [Crossref] [PubMed]
- Loganadane G, Dhermain F, Louvel G, et al. Brain Radiation Necrosis: Current Management With a Focus on Non-small Cell Lung Cancer Patients. Front Oncol 2018;8:336. [Crossref] [PubMed]
- Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 2008;25:51-8. [Crossref] [PubMed]
- Soussain C, Ricard D, Fike JR, et al. CNS complications of radiotherapy and chemotherapy. Lancet 2009;374:1639-51. [Crossref] [PubMed]
- Asao C, Korogi Y, Kitajima M, et al. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol 2005;26:1455-60. [PubMed]
- Sugahara T, Korogi Y, Tomiguchi S, et al. Posttherapeutic intraaxial brain tumor: the value of perfusion-sensitive contrast-enhanced MR imaging for differentiating tumor recurrence from nonneoplastic contrast-enhancing tissue. AJNR Am J Neuroradiol 2000;21:901-9. [PubMed]
- Winter SF, Vaios EJ, Muzikansky A, et al. Defining Treatment-Related Adverse Effects in Patients with Glioma: Distinctive Features of Pseudoprogression and Treatment-Induced Necrosis. Oncologist 2020;25:e1221-32. [Crossref] [PubMed]
- Xing S, Fan Z, Shi L, et al. Successful treatment of brain radiation necrosis resulting from triple-negative breast cancer with Endostar and short-term hyperbaric oxygen therapy: a case report. Onco Targets Ther 2019;12:2729-35. [Crossref] [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [Crossref] [PubMed]
- Xu Y, Rong X, Hu W, et al. Bevacizumab Monotherapy Reduces Radiation-induced Brain Necrosis in Nasopharyngeal Carcinoma Patients: A Randomized Controlled Trial. Int J Radiat Oncol Biol Phys 2018;101:1087-95. [Crossref] [PubMed]
- Ruben JD, Dally M, Bailey M, et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 2006;65:499-508. [Crossref] [PubMed]
- Park MS, Patel SR, Ludwig JA, et al. Activity of temozolomide and bevacizumab in the treatment of locally advanced, recurrent, and metastatic hemangiopericytoma and malignant solitary fibrous tumor. Cancer 2011;117:4939-47. [Crossref] [PubMed]
- Wang K, Mei F, Wu S, et al. Hemangiopericytoma: Incidence, Treatment, and Prognosis Analysis Based on SEER Database. Biomed Res Int 2020;2020:2468320. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Chmielecki J, Crago AM, Rosenberg M, et al. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet 2013;45:131-2. [Crossref] [PubMed]
- Stessin AM, Sison C, Nieto J, et al. The role of postoperative radiation therapy in the treatment of meningeal hemangiopericytoma-experience from the SEER database. Int J Radiat Oncol Biol Phys 2013;85:784-90. [Crossref] [PubMed]
- Lee JH, Jeon SH, Park CK, et al. The Role of Postoperative Radiotherapy in Intracranial Solitary Fibrous Tumor/Hemangiopericytoma: A Multi-institutional Retrospective Study (KROG 18-11). Cancer Res Treat 2022;54:65-74. [Crossref] [PubMed]
- Sonabend AM, Zacharia BE, Goldstein H, et al. The role for adjuvant radiotherapy in the treatment of hemangiopericytoma: a Surveillance, Epidemiology, and End Results analysis. J Neurosurg 2014;120:300-8. [Crossref] [PubMed]
- Chamberlain MC, Glantz MJ. Sequential salvage chemotherapy for recurrent intracranial hemangiopericytoma. Neurosurgery 2008;63:720-6; author reply 726-7. [Crossref] [PubMed]
- Baetta R, Soma M, De-Fraja C, et al. Upregulation and activation of Stat6 precede vascular smooth muscle cell proliferation in carotid artery injury model. Arterioscler Thromb Vasc Biol 2000;20:931-9. [Crossref] [PubMed]
- Martin-Broto J, Mondaza-Hernandez JL, Moura DS, et al. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers (Basel) 2021;13:2913. [Crossref] [PubMed]
- Domont J, Massard C, Lassau N, et al. Hemangiopericytoma and antiangiogenic therapy: clinical benefit of antiangiogenic therapy (sorafenib and sunitinib) in relapsed malignant haemangioperyctoma /solitary fibrous tumour. Invest New Drugs 2010;28:199-202. [Crossref] [PubMed]
- Zhuang H, Shi S, Yuan Z, et al. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer 2019;18:21. [Crossref] [PubMed]
- Wong ET, Huberman M, Lu XQ, et al. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol 2008;26:5649-50. [Crossref] [PubMed]
- Alessandretti M, Buzaid AC, Brandão R, et al. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep Oncol 2013;6:598-601. [Crossref] [PubMed]
- Vaios EJ, Batich KA, Buckley AF, et al. Resolution of radiation necrosis with bevacizumab following radiation therapy for primary CNS lymphoma. Oncotarget 2022;13:576-82. [Crossref] [PubMed]
- Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol 2013;15:1257-63. [Crossref] [PubMed]
- Zhuang H, Yuan X, Chang JY, et al. Exploration of the recurrence in radiation brain necrosis after bevacizumab discontinuation. Oncotarget 2016;7:48842-9. [Crossref] [PubMed]
Cite this article as: McKay MJ, Zadeh H, Foster R, Dumbrava M. Pathologically documented brain necrosis: response to bevacizumab after irradiation for solitary fibrous tumour/haemangiopericytoma: case report and literature review. Precis Cancer Med 2023;6:16.

