
A case report of EGFR L861Q mutation in synchronous de novo small cell and non-small cell lung cancer: molecular interrogation identifies origin and potential options for targeted therapy
Highlight box
Key findings
• Synchronous EGFR L861Q mutation within the SCLC and NSCLC biopsy results.
What is known and what is new?
• EGFR mutation commonly found within NSCLC.
• Unclear of the relevance and prevalence of an EGFR mutation in synchrony with a NSCLC and SCLC.
What is the implication, and what should change now?
• EGFR known as a driver mutation within NSCLC which could also be a therapeutic option if a synchronous SCLC with the same mutation is identified.
Introduction
Lung cancer remains the leading cause of cancer death in the United States. Non-small cell lung cancer (NSCLC) comprises 85% of cases and small cell lung cancer (SCLC) comprises most of the remainder (1). Targeted therapies have improved the prognosis of NSCLC; therefore, accurate determination of the genetic basis of lung cancer is essential for optimal management. Epidermal growth factor receptor (EGFR) mutations have been found in 26% of patients diagnosed with NSCLC (2) whereas EGFR mutations are altered in only 9.4% of patients with SCLC, and specifically L861Q has been rarely reported (2-5). EGFR exon 19 deletion mutation and EGFR exon 21 L858R point mutation are among the most commonly sensitizing mutations found. Transformation to SCLC occurs in 3–10% of EGFR-mutant NSCLC (5) and most cases of EGFR-mutated SCLC result from transformation, a scenario which may alter the approach to treatment (6). To our knowledge, there is no report of a shared EGFR L861Q mutation between two different sites representing NSCLC and SCLC. We present a patient with both NSCLC and SCLC, each harbouring the EGFR L861Q point mutation, and discuss the treatment implications of this mutation. We present this case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-67/rc).
Case presentation
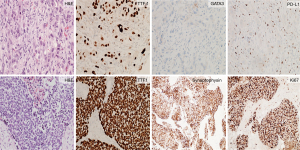
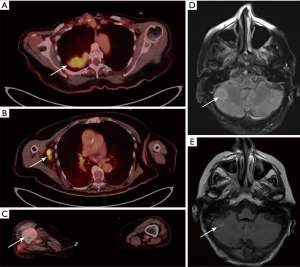
We present a case of a 78-year-old female with tobacco use in the past, who was found by screening mammogram to have right-sided bulky axillary lymphadenopathy and was referred to the hospital for expedited work-up. Core needle biopsy of a right axillary lymph node was performed. The morphology showed a poorly differentiated malignancy, and immunohistochemistry was consistent with metastatic non-small cell carcinoma (Figure 1: top). 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) demonstrated a 5.3 cm × 3.8 cm mass within the right lung, scattered pulmonary nodules, and lymphadenopathy in the chest, axillae, pelvis and retroperitoneum (Figure 2A,2B). 18F-FDG PET/CT imaging also showed a right distal femur mass (Figure 2C), which was subsequently biopsied and showed pathologic features consistent with metastatic small cell carcinoma (Figure 1: bottom). Magnetic resonance imaging (MRI) of the brain identified multiple numerous enhancing lesions suggestive of leptomeningeal metastasis (Figure 2D,2E).
Next-generation sequencing (NGS) of biopsies from the lymph node and femur was performed to determine the relatedness of these malignancies and facilitate management decisions. Molecular profiling showed shared mutations in multiple genes: PIK3CA, EGFR, TP53, NFKB1A, and NKX2-1 (Table 1). The patient completed 8/10 fractions of whole brain radiation as well as 5 fractions of radiation treatments to the right femur. The patient then started carboplatin AUC4 and etoposide 65 mg/m2 to be given every three weeks. Unfortunately, anti-EGFR therapy was not able to be initiated in this patient as after only two cycles of chemotherapy, the patient was hospitalized with altered mental status and the identification by MRI of new metastasis. The patient decided to pursue comfort care and hospice without further treatment of her malignancy.
Table 1
| Site | LN | Femur |
|---|---|---|
| Histology | Non-small cell carcinoma | Small-cell carcinoma |
| Shared mutations | PIK3CA H1047L; AF: LN, 7.8%; bone, 87.3% | |
| EGFR L861Q; AF: LN, 21.3%; bone, 83% | ||
| TP53 P250L; AF: LN, 17.3%; bone, 80.5% | ||
| NFKBIA amplification; AC: LN, 17; bone, 9 | ||
| NKX2-1 amplification; AC: LN, 28; bone, 10 | ||
| Unique mutations | BARD1 splice site 1315-1G>C; AF: 10.3% | RB1 splice site 2107-1G>T; AF: 78.5% |
| RAD21 amplification; AC: 12 | BCL2L2 amplification; AC: 9 | |
| AKT1 amplification; AC: 23 | ZNF217 amplification—equivocal; AC: 8 | |
| GNAS amplification—equivocal; AC: 8 | ||
| AURKA amplification—equivocal; AC: 8 | ||
| PDL-1 staining | <1% of tumor cells | Focal positivity in inflammatory/stromal cells |
AF, Allele frequencies; AC, amplification copies; LN, lymph node; PD-L1, programmed death-ligand 1.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The patient provided written consent for this case report prior to her passing; however the form was lost. Telephone consent obtained from next of kin, spouse, who is in full support of the publication of this article. Consent form indicating the next of kin including contact information is available for review by the editorial office of this journal.
Molecular tumor board discussion
The case was presented at the Northwell Health Molecular Tumor Board for discussion of the following consult questions.
Based on a review of the pathology, imaging, and molecular analysis, does the board believe the NSCLC and SCLC are more likely distinct primary malignancies or derived from a common origin? And, if the tumors share a common origin, are there any molecular features that would be consistent with transformation?
The tumor board discussed the possibility of combined SCLC with NSCLC component, each harboring the EGFR L861Q mutation, with the SCLC component metastasizing to the femur and the NSCLC component metastasizing to the axillary node. However, the consensus was that the two lung cancers had a common origin given the preponderance of shared mutations, including the rare EGFR L861Q mutation, as well as the known ability of NSCLC to transform to SCLC (Table 1).
On the topic of transformation, it was discussed that the femur biopsy demonstrated an RB1 mutation, with a 78.5% allele frequency which is likely the driver mutation for the SCLC transformation. Literature review of tumor samples and cell lines derived from resistant EGFR mutant patients demonstrated that retinoblastoma (RB) is lost in 100% of these SCLC transformed cases but rarely in those that remain NSCLC (7) RB1 inactivation is well known to play an important role in the tumorigenesis of SCLC (8,9). There have been many genomic alterations identified in SCLC including RB loss, c-Kit overexpression, telomerase activation, c-Myc amplification and p53 mutation; however, RB1 mutation or inactivation is a hallmark in SCLC (9). Therefore, the presence of the rare EGFR mutation and the presence of the RB1 mutation were supportive evidence of transformation. It is important to note however there are reports of EGFR-mutation positive SCLC occurring without any preceding diagnosis of NSCLC (5).
The allele frequency of the shared EGFR L861Q mutation was 83% in the SCLC biopsy and 21.3% in the NSCLC biopsy. A possible explanation for this finding is that the transformation of the SCLC derived from a subclone of the NSCLC containing this mutation, and the SCLC subsequently developed a copy number amplification or loss of heterozygosity of the EGFR gene.
What would be the predictive benefit for anti-EGFR directed therapy for this uncommon mutation?
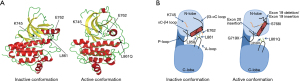
Tyrosine kinase inhibitors are effective treatments for NSCLCs with EGFR mutations. The EGFR L861Q missense mutation arises from the nucleotide change c.2582T>A in exon 21, resulting in a substitution to glutamine from leucine at position 861 (Figure 3A) (10). The EGFR-L861Q mutation confers conformational changes leading to an active kinase form. The Leu-861 is packed in a hydrophobic core of the wild-type structure and switching to a polar residue triggers a conformational transition of the activation loop folding outwards, towards an active-like kinase state (Figure 3) (10,11).
In general, EGFR mutations in the kinase domain EGFR activate the kinase, sensitizing patients to treatment with an EGFR inhibitor. Review of literature with this specific mutation, there is an associated response rate of 56% to afatinib and 40–60% with erlotinib or gefitinib (12-14) suggesting this patient may benefit from EGFR tyrosine kinase inhibitors. Early studies have shown that the L861Q mutation in particular displays enhanced kinase activity and transforming potential compared with other uncommon EGFR mutations, though it is not necessarily a drug-sensitizing mutation (15). Several clinical trials (16,17) have shown an intermediate response rate to afatinib, though largely in the NSCLC setting.
Studies of L861Q in the Ba/F3 model system demonstrated that similar to S768I mutations, L861Q is resistant to first-generation EGFRi compared to L858R, but unlike S768I, L861Q was sensitive to both afatinib and osimertinib treatment (18). The esophageal cancer cell line KYSE270 that harbors an endogenous EGFR L861Q mutation also showed comparable results to the Ba/F3 model system (19). These data suggest that both second- and third-generation inhibitors may be effective at targeting L861Q mutations (11). Current phase II studies are ongoing to evaluate the use of osimertinib in patients with L861Q mutations and initial reports are promising with 77% of patients achieving a partial response (20-22).
What would be the most appropriate management for this patient with the molecular findings?
Regarding the management plan, it was discussed whether imaging or molecular evidence could identify a substantial disparity in burden from either cancer type, which if found, might motivate treating the cancer type of greater burden. After extensive discussion at tumor board, the decision was made to proceed with treatment for the patient’s SCLC given the more aggressive nature of this disease in general compared to NSCLC. Data supporting combining tyrosine kinase inhibitors and chemotherapy are sparse in the context of EGFR mutation positive co-occurring NSCLC and SCLC. Many large-scale phases III randomized controlled trials (23,24) have shown that adding chemotherapy to EGFR-tyrosine kinase inhibitors (EGFR-TKIs) did not improve survival benefit in NSCLC. While this observation could be related to the fact that these studies were not specific to EGFR-mutant NSCLC, a possible explanation for lack of benefit could be because of G1-phase cell-cycle arrest by EGFR-TKI which may in turn result in subdued efficacy of chemotherapy. On the contrary, recent studies (25) a large meta-analysis demonstrated that EGFR-TKI combined with chemotherapy has superior efficacy compared to EGFR-TKI alone in advanced EGFR mutated NSCLC. Therefore, adding a TKI to chemotherapy is still controversial and ongoing trials will hopefully answer this question with more clarity. Nevertheless, the combination has been shown to be more toxic in multiple studies, compared to chemotherapy or TKI alone and for our patient with performance status (PS) of 2, we wanted to make sure to provide a treatment that was urgently needed with plans to add afatinib after completion of four cycles of chemotherapy.
Conclusions
Molecular interrogation is a valuable tool to identify shared origin of synchronous malignancies. This case demonstrates synchronous NSCLC and SCLC disease whose etiology was elucidated using molecular profiling as an RB1 mutation is often observed in transformed SCLC, and the rare EGFR mutation is not reported in de novo SCLC but is found, albeit uncommonly, in NSCLC.
EGFR is a well-studied driver mutation which can be targeted. This case had an EGFR L861Q mutation in which tyrosine kinase inhibitors that have shown efficacy. Given this patient had a mutation that can be targeted which was harbored in both SCLC and NSCLC, treatment options included the addition of targeted treatment along with chemotherapy. Unfortunately, the patient progressed prior to initiation of anti-EGFR therapy and pursued hospice. This case represents the importance of molecular profiling and testing especially when there are two different histologic pathologies. The case presented provides a platform for academic discussion and highlights the importance of molecular tumor board discussions which can assist with guiding treatment.
Acknowledgments
Thanks for Northwell Health and the Northwell Health Molecular Tumor Board for their support in publishing this work.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-67/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-67/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-67/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). The patient provided written consent for this case report prior to her passing; however the form was lost. Telephone consent obtained from next of kin, spouse, who is in full support of the publication of this article. Consent form indicating the next of kin including contact information is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov 2017;7:818-31. [Crossref] [PubMed]
- Takagi Y, Nakahara Y, Hosomi Y, et al. Small-cell lung cancer with a rare epidermal growth factor receptor gene mutation showing "wax-and-wane" transformation. BMC Cancer 2013;13:529. [Crossref] [PubMed]
- O'Kane GM, Bradbury PA, Feld R, et al. Uncommon EGFR mutations in advanced non-small cell lung cancer. Lung Cancer 2017;109:137-44. [Crossref] [PubMed]
- Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278-85. [Crossref] [PubMed]
- Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013;8:1265-71. [Crossref] [PubMed]
- Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-e172. [Crossref] [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [Crossref] [PubMed]
- Dixit A, Yi L, Gowthaman R, et al. Sequence and structure signatures of cancer mutation hotspots in protein kinases. PLoS One 2009;4:e7485. [Crossref] [PubMed]
- Harrison PT, Vyse S, Huang PH. Rare epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol 2020;61:167-79. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Wu JY, Yu CJ, Chang YC, et al. Effectiveness of tyrosine kinase inhibitors on "uncommon" epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res 2011;17:3812-21. [Crossref] [PubMed]
- Chiu CH, Yang CT, Shih JY, et al. Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Treatment Response in Advanced Lung Adenocarcinomas with G719X/L861Q/S768I Mutations. J Thorac Oncol 2015;10:793-9. [Crossref] [PubMed]
- Kancha RK, Peschel C, Duyster J. The epidermal growth factor receptor-L861Q mutation increases kinase activity without leading to enhanced sensitivity toward epidermal growth factor receptor kinase inhibitors. J Thorac Oncol 2011;6:387-92. [Crossref] [PubMed]
- Joshi M, Rizvi SM, Belani CP. Afatinib for the treatment of metastatic non-small cell lung cancer. Cancer Manag Res 2015;7:75-82. [Crossref] [PubMed]
- Sharma N, Graziano S. Overview of the LUX-Lung clinical trial program of afatinib for non-small cell lung cancer. Cancer Treat Rev 2018;69:143-51. [Crossref] [PubMed]
- Banno E, Togashi Y, Nakamura Y, et al. Sensitivities to various epidermal growth factor receptor-tyrosine kinase inhibitors of uncommon epidermal growth factor receptor mutations L861Q and S768I: What is the optimal epidermal growth factor receptor-tyrosine kinase inhibitor? Cancer Sci 2016;107:1134-40. [Crossref] [PubMed]
- Yang Y, Tian Z, Zhao X, et al. A novel antitumor dithiocarbamate compound inhibits the EGFR/AKT signaling pathway and induces apoptosis in esophageal cancer cells. Oncol Lett 2020;20:877-83. [Crossref] [PubMed]
- Phase II Study of Afatinib Plus Bevacizumab in the Treatment Epidermal Growth Factor Receptor (EGFR) Exon G719X, S768I, and L861Q Mutation Metastatic Non-Small Cell Lung Cancer - Full Text View - ClinicalTrials.gov [Internet]. [cited 2023 Apr 7]. Available from: https://clinicaltrials.gov/ct2/show/NCT05267288
- Takeda M, Shimokawa M, Nakamura A, et al. A phase II study (WJOG12819L) to assess the efficacy of osimertinib in patients with EGFR mutation-positive NSCLC in whom systemic disease (T790M-negative) progressed after treatment with first- or second-generation EGFR TKIs and platinum-based chemotherapy. Lung Cancer 2023;177:44-50. [Crossref] [PubMed]
- Sehgal K, Rangachari D, VanderLaan PA, et al. Clinical Benefit of Tyrosine Kinase Inhibitors in Advanced Lung Cancer with EGFR-G719A and Other Uncommon EGFR Mutations. Oncologist 2021;26:281-7. [Crossref] [PubMed]
- Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol 2004;22:777-84. [Crossref] [PubMed]
- Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 2004;22:785-94. [Crossref] [PubMed]
- Wu Q, Luo W, Li W, et al. First-Generation EGFR-TKI Plus Chemotherapy Versus EGFR-TKI Alone as First-Line Treatment in Advanced NSCLC With EGFR Activating Mutation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Oncol 2021;11:598265. [Crossref] [PubMed]
Cite this article as: Hines AM, Patruni S, Kataria N, Boyd J, Seetharamu N, King DA. A case report of EGFR L861Q mutation in synchronous de novo small cell and non-small cell lung cancer: molecular interrogation identifies origin and potential options for targeted therapy. Precis Cancer Med 2023;6:17.

