First-line ALK inhibitors in treatment-naive advanced ALK rearranged non-small cell lung cancer: systematic review and network meta-analysis
Highlight box
Key findings
• Lorlatinib had the greatest IRC PFS benefit compared with other ALK TKIs, and alectinib was superior for OS.
• In patients with baseline brain metastases, lorlatinib demonstrated the greatest IRC PFS benefit.
What is known and what is new?
• There are numerous first-line options for ALK TKI in metastatic ALK rearranged NSCLC.
• We conducted a network meta-analysis of nine RCTs to compare the relative efficacy and toxicity of six ALK TKIs.
What is the implication, and what should change now?
• In real-world clinical practice, numerous additional clinical considerations may also influence the selection of upfront ALK TKI.
Introduction
Background
Rearrangements in anaplastic lymphoma kinase (ALK) are detected in approximately 3–7% of patients with advanced non-small cell lung cancer (NSCLC) (1). Crizotinib initially demonstrated superiority compared to platinum-pemetrexed chemotherapy (2), establishing ALK tyrosine kinase inhibitors (TKI) as standard of care in the treatment-naïve metastatic setting. Subsequently, with the emergence of next generation ALK TKI targeted therapies, there have been phase III randomized controlled trials (RCTs) for compounds including alectinib, brigatinib, ceritinib, ensartinib and lorlatinib, each demonstrating improvements in outcomes over the standard of care control arm. Consequently, there is an expanding list of first-line therapeutic options and increasing complexity in optimally selecting and sequencing therapies (3). In addition to primary efficacy outcomes such as response and duration of response, the intracranial efficacy, toxicity profile and potential mechanisms of resistance to sequence therapies are all relevant considerations in selecting upfront therapy. In particular, for advanced ALK rearranged NSCLC, there is a high incidence of central nervous system (CNS) metastases at diagnosis, and the enhanced intracranial efficacy of next generation ALK TKI may allow for radiation therapy to be deferred (4).
Rationale and knowledge gap
Notably however, there are no phase III RCTs directly comparing next-generation ALK TKIs head-to-head. A network meta-analysis (NMA) allows for the comparison of multiple interventions to establish relative efficacy with both direct and indirect comparisons, in contrast to a standard pair-wise meta-analysis (5).
Objective
Therefore, we sought to conduct a systematic review and NMA to compare the efficacy of different ALK TKIs in treatment-naïve patients with ALK rearranged advanced NSCLC. The systematic review and NMA was conducted according to the PRISMA reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-54/rc) (6) and was prospectively registered in PROSPERO (CRD42021250472).
Methods
Eligibility criteria
Phase III RCTs were included if they had a full-text publication in English. Eligible trials were conducted in treatment-naive ALK rearranged advanced NSCLC patients and compared an ALK TKI (either alone or in combination) with standard of care therapy (either another ALK inhibitor or chemotherapy). Outcomes of interest included progression-free survival (PFS) by independent review criteria (IRC) and investigator assessed (IA), overall survival (OS), IRC PFS for patients both with and without baseline brain metastases, objective response rate (ORR), intracranial response rate for patients both with and without baseline brain metastases, and toxicities. Other data variables collected included number of participants in each arm, dose interruptions, dose reductions, dose discontinuations and treatment-related deaths.
Search strategy
A comprehensive literature search was performed in MEDLINE from inception until May 2022 utilising for RCTs using search terms such as ‘ALK fusion’, ‘ALK inhibitor’, ‘ALK rearrangement’, ‘ALK tyrosine kinase inhibitor’, ‘lung cancer’ and ‘non-small cell lung cancer’. Filters were utilized to select for RCTs where possible. The grey literature was also searched including ClinicalTrials.gov and references from published papers.
Study selection
All studies were identified and reviewed by two individual reviewers (AC Tan, SH Tan) with review of abstracts and full-text articles where appropriate. Disagreements were resolved by consensus.
Data extraction and risk of bias
Prespecified study characteristics and data were extracted by one reviewer (SH Tan) and verified by a second reviewer (AC Tan), with disagreements resolved by discussion or referred to a third reviewer (DSW Tan). Risk of bias was assessed using the Cochrane Collaboration’s tool by two individual reviewers (AC Tan, SH Tan), with disagreements resolved by discussion or referred to a third reviewer (DSW Tan).
Statistical analysis
Bayesian fixed-effects NMA and pairwise meta-analysis (MA) were performed to generate estimates of all possible pair-wise comparisons within the network for IRC PFS, IA PFS, OS, IRC PFS for patients both with and without baseline brain metastases, ORR, intracranial response rate for patients both with and without baseline brain metastases, and toxicities. For toxicities, proportions of patients with Common Terminology Criteria for Adverse Events (CTCAE) grade 3 or higher all-cause adverse events, rates of dose reduction due to adverse events, rates of dose discontinuation due to adverse events and commonly reported specific adverse events were evaluated. Random-effects pairwise meta-analysis was initially used to assess the between-study heterogeneity. For the final NMA, the fixed-effect model in which the same true effect size was assumed for all trials was utilised. The fixed-effect NMA model was selected because most of the treatment comparisons were evaluated in a single trial and the total number of trials included in the network was too small to appropriately estimate the between-study heterogeneity. Non-informative uniform and normal prior distributions were used and three different sets of initial values were used to fit the model. Convergence of the three sets of iterations were assessed by visual inspection of the three chains to ensure the convergence of the parameter estimates and in accordance with the Brooks-Gelman-Rubin diagnostic and autocorrelation was assessed using autocorrelation plot. Once convergence was established, the posterior distribution summary statistics of the model were reported as the results of the NMA. Hazard ratios (HR) or odds ratios (OR) and 95% credible intervals (CrI; or Bayesian intervals analogous to Frequentist confidence intervals) are reported for each pair-wise comparison.
Different doses of the same drug (alectinib), and platinum-pemetrexed chemotherapy (with or without maintenance pemetrexed) were combined into single nodes to complete the analysis. The surface under the cumulative ranking curve (SUCRA) (7) is provided for all outcomes to determine the overall ranking of each treatment. SUCRA is a numeric presentation of the overall ranking and presents a percentage (ranging from 0% to 100%) associated with each treatment, the higher the SUCRA value (closer to 100%), the higher the likelihood that the treatment is top rank or one of the top ranks. For toxicity evaluation, a higher SUCRA value represented a therapy with less toxicity or lower rates of dose reduction or discontinuation. Fixed-effects MA and NMA models were implemented using Markov chain Monte Carlo (MCMC) simulations in WinBUGS (MRC Biostatistics Unit) (8) with 50,000 MCMC iterations and 50,000 burn-ins (iterations that were discarded) with a thinning interval of 10. Results were processed, tabulated and graphical plots (9) were produced using R statistical software (R Project for Statistical Computing) (10). Two key assumptions underlying NMA are transitivity and consistency; transitivity relates to the exchangeability across studies to allow for the comparison of two treatments via a third treatment, while consistency considers if the direct and indirect estimates are statistically similar. Inconsistency between direct and indirect evidence on a particular pairwise comparison was assessed using the node splitting approach (11) if there were closed loops in the NMA; otherwise for network without closed loops, we assess exchangeability by comparing the study and patients’ characteristics to ensure that they satisfied the assumption that all patients were equally likely to receive the given treatments in the network. Transitivity was managed by the inclusion of RCTs with strict patient selection and allocation to address all treatments for the same condition. It is evaluated by using descriptive statistics of study baseline variables, such as age, gender and sample size.
Results
Study selection and characteristics
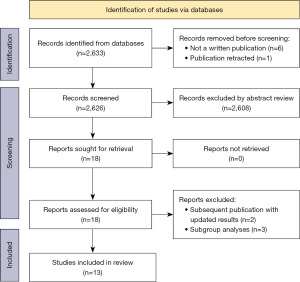
A total of 2,633 records were identified, of which 13 publications reporting results from 9 RCTs were eligible and included (Figure 1; Table S1). The intervention arm in the trials included crizotinib [versus chemotherapy in PROFILE 1014 (2,12) and PROFILE 1029 (13)], alectinib [versus crizotinib in ALEX (14,15), ALESIA (16) and J-ALEX (17-19)], brigatinib [versus crizotinib in ALTA-1L (20-22)], ceritinib [versus chemotherapy in ASCEND-4 (23)], ensartinib [versus crizotinib in eXalt3 (24)] and lorlatinib [versus crizotinib in CROWN (25,26)]. The distribution of potential effect modifiers such as age, gender and proportion of patients with brain or CNS metastases were comparable across all trials (Table S1).
Network meta-analysis
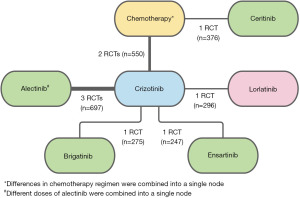
For IRC PFS and OS, there were 9 RCTs (Figure 2), and for IA PFS, there were 4 RCTs (Figure S1). The analysis of OS data for ALESIA, ASCEND-4, eXalt3 and CROWN were based on immature OS results. OS and IA PFS outcomes in the chemotherapy naïve subgroups for ALTA-1L and J-ALEX were not reported, and were therefore excluded from these analyses. Similarly, outcomes for patients with and without CNS metastases stratified by prior chemotherapy were not reported separately in ALTA-1L, and was therefore excluded from this analysis. Due to the lack of IA PFS outcomes for PROFILE 1014 and PROFILE 1029, there was no link between crizotinib and chemotherapy, resulting in a broken link with ceritinib. Consequently, ASCEND-4 was excluded from the IA PFS evidence network. Intracranial or CNS response was not reported for PROFILE 1014 and PROFILE 1029 resulting in a broken link with ceritinib for the intracranial response evidence network. Rates of dose reduction due to adverse events was not reported for PROFILE 1014 and PROFILE 1029 resulting in a broken link with ceritinib for the dose reduction evidence network.
Comparisons of PFS, ORR & OS in the overall study populations
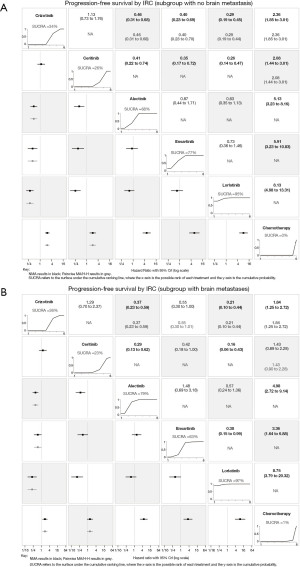
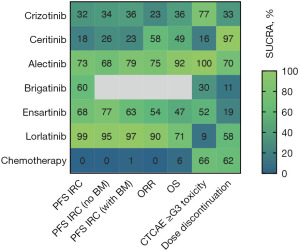
Pair-wise comparisons and NMA IRC PFS and OS HR with 95% CrI for all TKI and chemotherapy are presented in Figure 3. For IRC PFS (Figure 3A), all included ALK TKIs were found to be superior to chemotherapy. Lorlatinib showed IRC PFS benefit compared with all other ALK TKIs, which was reflected by the highest cumulative ranking curve SUCRA of 99%. However, the pair-wise comparisons with ensartinib approached, but was not statistically significant, with NMA IRC PFS HR of 0.60 (95% CrI: 0.35–1.03). Similarly for IA PFS (Figure S2), lorlatinib had the highest SUCRA (100%). The pair-wise comparison showed superiority for lorlatinib compared with alectinib, with NMA IA PFS HR of 0.52 (95% CrI: 0.33–0.81). For ORR (Figure S3A), again all ALK TKIs were superior to chemotherapy with NMA ORR OR ranging from 0.09 (95% CrI: 0.05–0.16) for lorlatinib to 0.21 (95% CrI: 0.14–0.31) for crizotinib. Lorlatinib (90%) had the highest SUCRA for ORR. However, pair-wise comparisons for lorlatinib with other ALK TKIs were not significantly different apart from crizotinib.

In regards to OS (Figure 3B), all ALK TKIs appear to be superior to chemotherapy. However, only alectinib was statistically significant, with NMA OS HR of 2.14 (95% CrI: 1.38–3.33). Pair-wise comparisons between ALK TKIs demonstrated superior OS outcomes for alectinib and lorlatinib compared with other ALK TKIs. Alectinib had the highest SUCRA for OS outcomes (92%) closely followed by lorlatinib (71%).
Comparisons of PFS and intracranial response according to the presence of baseline brain metastases
Notably, in patients without baseline brain metastases, all ALK TKIs were statistically superior to chemotherapy (Figure 4A). Among ALK TKIs, lorlatinib (95%) had the highest SUCRA followed by ensartinib (77%) and alectinib (68%). In patients with baseline brain metastases, all ALK TKIs apart from ceritinib were superior to chemotherapy (Figure 4B). Similarly, lorlatinib (97%) had the highest SUCRA although followed by alectinib (79%). Pair-wise comparisons, however, showed no statistically significant difference between lorlatinib and alectinib in both the subgroups for patients without and with baseline brain metastases.
For intracranial response in patients with measurable baseline brain metastases (Figure S3B), included ALK TKIs (alectinib, ensartinib and lorlatinib) were superior to crizotinib. Lorlatinib (84%) had the highest SUCRA, followed by alectinib (61%). In patients with both measurable or non-measurable baseline brain metastases (Figure S3C), lorlatinib (88%) had the highest SUCRA—although notably brigatinib, ceritinib and ensartinib were not included in this evidence network.
Comparisons of toxicity in the overall study populations
Toxicity and safety were evaluated by comparing the ratio of the odds of patients experiencing (to not experiencing) CTCAE grade 3 or higher all-cause adverse events, dose reduced due to treatment-related adverse events and discontinued treatment due to adverse events between ALK TKIs (Figure S4, Table S2). Alectinib (100%) had the highest SUCRA, and was superior to all other ALK TKIs and chemotherapy with regards to the lowest odds of experiencing grade 3 or higher adverse events. In regards to dose discontinuation, ceritinib (97%) followed by alectinib (70%), chemotherapy (62%) and lorlatinib (58%) had the highest SUCRA indicating the lowest odds of requiring dose discontinuation due to adverse events. Commonly reported specific adverse events were also compared across treatments as shown in Table S3. This demonstrated toxicities most likely to be associated with specific ALK TKIs such as diarrhoea, vomiting, nausea and loss of appetite (all SUCRA 0–1%) with ceritinib, vision disorder (0%) with crizotinib, rash (1%) with ensartinib and oedema (1%) with lorlatinib.
Risk of bias and certainty of evidence
The risk of bias assessment is shown in Table S4 and summarized in Table S5. Overall, the trials were assessed to be at low risk of bias, with the exception of performance bias and detection bias for subjective outcomes due to the open-label design of the included trials. However, the blinded independent review of treatment response significantly lowers the risk of detection bias for objective outcomes. We also used the GRADE approach to rate the certainty of evidence for each outcome measure (Table S6).
Discussion
Key findings
There are now multiple ALK TKIs which have demonstrated survival benefit in phase III RCTs. A lack of head-to-head comparisons, particularly between next-generation ALK TKIs however, have resulted in significant debate over the selection of optimal upfront therapy (27,28). In this NMA, pair-wise comparisons allowed for direct and indirect comparisons between different ALK TKIs (Figure 5). Lorlatinib had the highest SUCRA with respect to IRC PFS, whilst alectinib had the highest SUCRA in terms of OS.
Strengths and limitations
By its very nature, a NMA conducts either an indirect comparison or the synthesis of direct and indirect comparisons, and findings should be interpreted extremely carefully in this context. In addition, there were several broken links, and the link for lorlatinib with crizotinib is limited to one trial compared to alectinib with three trials. Heterogeneity amongst trials, such as patient baseline characteristics including ethnicity and differences in study protocols may distort an indirect comparison (intransitivity) and are other potential limitations. Furthermore, in our analysis, study-level data rather than individual patient data was utilised. Nevertheless, with formal statistical comparisons our study provides important context for the therapeutic landscape of treatment naïve ALK rearranged advanced NSCLC.
Importantly in our study, ALK TKIs (alectinib, brigatinib, crizotinib, ensartinib and lorlatinib) demonstrated superiority with regards to IRC PFS over chemotherapy. In addition, next-generation ALK TKIs (alectinib, brigatinib, ensartinib and lorlatinib) demonstrated improved IRC PFS compared to first-generation ALK TKI crizotinib, reflected in the increasing number of approvals globally. Differences in the chemotherapy arm for ASCEND-4, with the allowed use of maintenance pemetrexed, likely influenced the comparison of ceritinib with crizotinib and the remaining ALK TKIs. Lorlatinib was superior to other next-generation ALK TKIs for ORR, IRC PFS and IRC PFS in patients with and without brain metastases. This suggests lorlatinib represents the optimal first-line therapeutic option with regards to efficacy, however costs and toxicities are important considerations. With regards to OS, alectinib followed by lorlatinib had the highest probabilities of being the best treatment, although OS outcomes in most studies—including ALESIA, ALTA-1L and CROWN—remains immature. Conversely, the sequencing of therapies may also partially explain this finding, given the demonstrated efficacy for lorlatinib after resistance to one or more prior ALK TKIs (29).
The optimal selection of ALK TKI in the upfront setting remains complex, and despite the superiority of lorlatinib with respect to IRC PFS based on SUCRA, there are limitations in interpreting and applying SUCRA rankings to clinical practice (30). In addition, there are numerous other practical considerations. Crucially, there are differences in toxicity profiles of ALK TKIs, and in particular the neurocognitive adverse effects associated with lorlatinib are an important consideration (25). In our analysis, alectinib was associated with lower proportions of patients experiencing grade 3 or higher adverse events. However, differences in doses used in J-ALEX may have influenced this finding. For ceritinib, subsequent trials have also demonstrated the improved tolerability of lower doses when administered with food whilst maintaining efficacy (31). Distinct toxicity profiles were also illustrated in our analysis when considering commonly reported specific adverse events, particularly for ceritinib, crizotinib, ensartinib and lorlatinib. However, there remains further unique toxicities which were not included in our analysis but are still highly relevant in real-world clinical practice. For example, the neurocognitive toxicities, hyperlipidemia/hypertriglyceridemia and weight gain with lorlatinib, interstitial lung disease with brigatinib and hyperbilirubinaemia with alectinib.
The propensity for ALK rearranged NSCLC patients to develop brain metastases is well established, and intracranial efficacy between ALK TKIs may also differ (32). In our analysis, lorlatinib followed by alectinib were superior with regards to PFS in patients with baseline brain metastases. In contrast, for PFS in patients without baseline brain metastases, SUCRA rankings demonstrated superiority for lorlatinib, followed by ensartinib and alectinib. Moreover, there is heterogeneity even within ALK rearranged NSCLC, and different ALK fusion variants may influence responses to therapy (33). The method of detection of ALK rearrangement with IHC, FISH and/or NGS may further impact therapeutic efficacy (34). Finally, there is growing evidence describing unique profiles for mechanisms of resistance (including primary resistance) to different ALK TKIs with varying rates of ALK resistance mutations and off-target pathway activation such as MET amplification (35,36)—which may also influence the selection and sequencing of therapies. This highlights the ongoing need for improved therapeutic strategies, despite durable responses in most patients.
Comparison with similar researches
Several meta-analyses or NMA of ALK TKIs have been reported previously, demonstrating the relative efficacy of ALK TKIs compared to chemotherapy (37), or alectinib (38) and lorlatinib (39) compared to other ALK TKIs (40-42). However, crucially in our study and in contrast to prior reports, we included trials of chemotherapy allowing for comparisons with ceritinib, only included randomized phase III trials, the final results from the ALTA-1L and J-ALEX trials, updated outcomes for CROWN, and data from the full publications for the CROWN and eXalt3 trials.
Conclusions
In conclusion, through formal statistical comparisons in a NMA, we demonstrated the superiority of next-generation ALK TKIs to chemotherapy and crizotinib. Lorlatinib had the highest probability of PFS benefit in comparing between next-generation ALK TKIs, however in real-world clinical practice, numerous additional clinical considerations may also influence the selection of upfront ALK TKI.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-54/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-54/coif). ACT receives consulting fees from Amgen, Bayer & Pfizer, and honoraria from ThermoFisher; and was the recipient of an International Association for the Study of Lung Cancer (IASLC) Fellowship 2018–2020. DSWT receives consulting fees from Novartis, Merck, Loxo Oncology, AstraZeneca, Roche, Pfizer, and honoraria from Bristol-Myers Squibb, Takeda Pharmaceuticals, Novartis, Roche and Pfizer; and is the recipient of an NMRC clinician-scientist award (NMRC/CSA/007/2016; NMRC/CSAINV19NOV-0005), and was supported in part by NMRC/OFLCG/002-2018. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018;15:694-708. [Crossref] [PubMed]
- Thomas NJ, Myall NJ, Sun F, et al. Brain Metastases in EGFR- and ALK-Positive NSCLC: Outcomes of Central Nervous System-Penetrant Tyrosine Kinase Inhibitors Alone Versus in Combination With Radiation. J Thorac Oncol 2022;17:116-29. [Crossref] [PubMed]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24. [Crossref] [PubMed]
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. [Crossref] [PubMed]
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71. [Crossref] [PubMed]
- Lunn DJ, Thomas A, Best N, et al. WinBUGS - A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing 2000;10:325-37. [Crossref]
- Tan SH, Cooper NJ, Bujkiewicz S, et al. Novel presentational approaches were developed for reporting network meta-analysis. J Clin Epidemiol 2014;67:672-80. [Crossref] [PubMed]
- R. Core Team. R: A language and environment for statistical computing. 2013. Available online: http://www.R-project.org/
- Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44. [Crossref] [PubMed]
- Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. [Crossref] [PubMed]
- Wu YL, Lu S, Lu Y, et al. Results of PROFILE 1029, a Phase III Comparison of First-Line Crizotinib versus Chemotherapy in East Asian Patients with ALK-Positive Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1539-48. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Mok T, Camidge DR, Gadgeel SM, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol 2020;31:1056-64. [Crossref] [PubMed]
- Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med 2019;7:437-46. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Nakagawa K, Hida T, Nokihara H, et al. Final progression-free survival results from the J-ALEX study of alectinib versus crizotinib in ALK-positive non-small-cell lung cancer. Lung Cancer 2020;139:195-9. [Crossref] [PubMed]
- Yoshioka H, Hida T, Nokihara H, et al. Final OS analysis from the phase III j-alex study of alectinib (ALC) versus crizotinib (CRZ) in Japanese ALK-inhibitor naïve ALK-positive non-small cell lung cancer (ALK+ NSCLC). J Clin Oncol 2021;39:9022. [Crossref]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J Clin Oncol 2020;38:3592-603. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in ALK Inhibitor-Naive Advanced ALK-Positive NSCLC: Final Results of Phase 3 ALTA-1L Trial. J Thorac Oncol 2021;16:2091-108. Erratum in: J Thorac Oncol 2022 doi: 10.1016/j.jtho.2022.07.009. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Horn L, Wang Z, Wu G, et al. Ensartinib vs Crizotinib for Patients With Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol 2021;7:1617-25. [Crossref] [PubMed]
- Shaw AT, Bauer TM, de Marinis F, et al. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med 2020;383:2018-29. [Crossref] [PubMed]
- Solomon BJ, Bauer T, Mok T, et al. Updated efficacy and safety from the phase 3 CROWN study of first-line lorlatinib vs crizotinib in advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC). AACR Annual Meeting 2022, Abstract #CT223.
- Camidge DR. Lorlatinib Should Not be Considered as the Preferred First-Line Option in Patients With Advanced ALK Rearranged NSCLC. J Thorac Oncol 2021;16:528-31. [Crossref] [PubMed]
- Nagasaka M, Ou SI. Lorlatinib Should Be Considered as the Preferred First-Line Option in Patients With Advanced ALK-Rearranged NSCLC. J Thorac Oncol 2021;16:532-6. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Mbuagbaw L, Rochwerg B, Jaeschke R, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:79. [Crossref] [PubMed]
- Cho BC, Obermannova R, Bearz A, et al. Efficacy and Safety of Ceritinib (450 mg/d or 600 mg/d) With Food Versus 750-mg/d Fasted in Patients With ALK Receptor Tyrosine Kinase (ALK)-Positive NSCLC: Primary Efficacy Results From the ASCEND-8 Study. J Thorac Oncol 2019;14:1255-65. [Crossref] [PubMed]
- Felip E, Shaw AT, Bearz A, et al. Intracranial and extracranial efficacy of lorlatinib in patients with ALK-positive non-small-cell lung cancer previously treated with second-generation ALK TKIs. Ann Oncol 2021;32:620-30. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated Efficacy and Safety Data and Impact of the EML4-ALK Fusion Variant on the Efficacy of Alectinib in Untreated ALK-Positive Advanced Non-Small Cell Lung Cancer in the Global Phase III ALEX Study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Mok T, Peters S, Camidge DR, et al. Outcomes According to ALK Status Determined by Central Immunohistochemistry or Fluorescence In Situ Hybridization in Patients With ALK-Positive NSCLC Enrolled in the Phase 3 ALEX Study. J Thorac Oncol 2021;16:259-68. [Crossref] [PubMed]
- Katayama R. Drug resistance in anaplastic lymphoma kinase-rearranged lung cancer. Cancer Sci 2018;109:572-80. [Crossref] [PubMed]
- Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res 2020;26:2535-45. [Crossref] [PubMed]
- Breadner D, Blanchette P, Shanmuganathan S, et al. Efficacy and safety of ALK inhibitors in ALK-rearranged non-small cell lung cancer: A systematic review and meta-analysis. Lung Cancer 2020;144:57-63. [Crossref] [PubMed]
- Elliott J, Bai Z, Hsieh SC, et al. ALK inhibitors for non-small cell lung cancer: A systematic review and network meta-analysis. PLoS One 2020;15:e0229179. [Crossref] [PubMed]
- Chuang CH, Chen HL, Chang HM, et al. Systematic Review and Network Meta-Analysis of Anaplastic Lymphoma Kinase (ALK) Inhibitors for Treatment-Naïve ALK-Positive Lung Cancer. Cancers (Basel) 2021;13:1966. [Crossref] [PubMed]
- Ma HC, Liu YH, Ding KL, et al. Comparative efficacy and safety of first-line treatments for advanced non-small cell lung cancer with ALK-rearranged: a meta-analysis of clinical trials. BMC Cancer 2021;21:1278. [Crossref] [PubMed]
- Ando K, Akimoto K, Sato H, et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer With or Without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers (Basel) 2020;12:942. [Crossref] [PubMed]
- Cameron LB, Hitchen N, Chandran E, et al. Targeted therapy for advanced anaplastic lymphoma kinase (<I>ALK</I>)-rearranged non-small cell lung cancer. Cochrane Database Syst Rev 2022;1:CD013453. [PubMed]
Cite this article as: Tan AC, Tan SH, Ang MK, Tan DSW. First-line ALK inhibitors in treatment-naive advanced ALK rearranged non-small cell lung cancer: systematic review and network meta-analysis. Precis Cancer Med 2023;6:3.