Endoscopic endonasal removal of nasal cavity chondrosarcoma extending to the base of the skull: a case report
Highlight box
Key findings
• Chondrosarcoma of the nasal cavity is a rare malignant tumor of cartilage tissue;
• Treatment of craniofacial chondrosarcomas consists of the most radical surgical removal and adjuvant radiation therapy;
• Endoscopic endonasal removal of the nasal cavity chondrosarcoma may be the method of choice even for large tumors spreading to the base of the skull.
What is known and what is new?
• Open transcranial and transfacial approaches are widely used in the treatment of craniofacial chondrosarcomas;
• Our case complements the general modest experience in the use of endoscopic endonasal removal of nasal cavity chondrosarcomas.
What is the subtext and what should change now?
• The introduction of a less traumatic endoscopic endonasal approach in the treatment of chondrosarcomas of the nasal cavity allows to accelerate recovery. Due to this, patients can start radiation therapy at an earlier time, which reduces the risk of recurrences.
Introduction
Chondrosarcoma is a malignant tumor that accounts for 8% of all head and neck sarcomas and only 0.1% of all head and neck malignancies (1).
There are five subtypes of chondrosarcomas: grades I–III, mesenchymal, and myxoid (2). The pathological classification of classes I–III subtypes is based on differences in differentiation: class I (well differentiated), class II (moderately differentiated), and class III (poorly differentiated), while tumors of the mesenchymal subtype have primitive spindle-shaped cells, and tumors of the myxoid type consist of rows of rounded cells (3).
The prognosis is based on the pathological classification. Five-year relapse-free survival rates in subtypes I, II and III, mesenchymal and myxoid subtypes are 15%, 16%, 33%, 63% and 16%, respectively, and 5-year survival rates are 90–95%, 81–90%, 43–75%, 46% and 94%, respectively (3,4).
The main treatment for chondrosarcomas of the nasal cavity is microsurgical resection usually performed via open transcranial and transfacial approaches (4,5). Over the last decade, an endoscopic endonasal method has been used increasingly for the treatment of skull base tumors. However, an endoscopic endonasal resection of chondrosarcomas of the nasal cavity has been rarely reported (6-9).
In the present article, we describe our experience in treating a patient with chondrosarcoma of the nasal cavity extending into the ethmoidal labyrinth, both orbits, both maxillary sinuses, hard palate. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-34/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
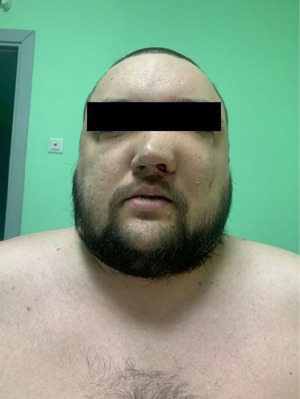
A 32-year-old patient presented to P. Hertsen Moscow Oncology Research Institute with complaints of the nasal blockage, bony deformity of the left zygomatic region. The patient first noticed the nasal blockage about two years ago. On clinical examination, there was a scanty mucohemorrhagic discharge from the left nasal passage. The left nasal cavity was partially obstructed by tumor (Figure 1).
The patient past medical history revealed that 16 years ago, the patient had a chondroma of the left maxillary sinus removed. The patient did not visit doctors after the surgery, no medical documentation was kept.
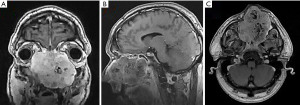
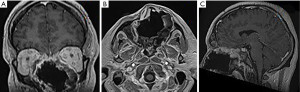
Provided contrast-enhanced magnetic resonance imaging (MRI) revealed a tumor of the nasal cavity extending to the ethmoidal labyrinth, both orbits, both maxillary sinuses, and a destruction of the hard palate (Figure 2).
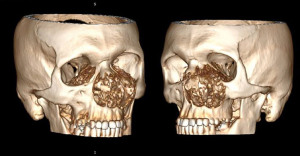
Spiral computed tomography (CT) of the skull bones revealed gross osteodestruction of the maxilla predominantly on the left, inferior medial walls of both orbits, the hard palate (Figure 3).
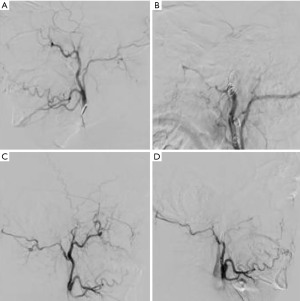
Considering the gigantic size of the tumor, it was decided to perform preoperative endovascular embolization of the vessels of the tumor stroma and parenchyma. The main sources of blood supply to the tumor were the branches of the maxillary arteries on both sides. Occlusion was performed using embolization coils. Control angiography revealed complete occlusion of the vessels feeding the tumor (Figure 4).
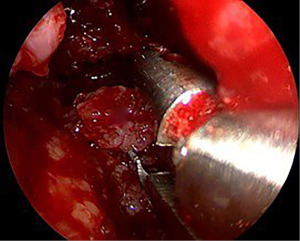
The main surgery began 1 day after embolization. Course of the surgery: external lumbar drain was placed. A high (cartilaginous) density moderately bleeding tumor was found during the surgery. In course of the tumor removal under the control of 0- and 30-degree endoscopes, it was revealed that the septum of the nasal cavity, as well as all turbinates on the left, had been completely destroyed by the tumor. We used 4-mm rigid nasal endoscopes. Removal was performed using conchotomes and suctions of various configurations (Figure 5). At the first stage, the tumor was removed from the nasal cavity, then from the hard palate infiltrated by the tumor, both maxillary sinuses, orbits, and the ethmoidal labyrinth under the control of a 45-degree endoscope.
Intact cells of the ethmoidal labyrinth and the mucous membrane of the sphenoid sinus, periosteum of the orbits were visualized after tumor removal. Endoscopic inspection revealed no residual tumor. Upon completion of the surgery, the lumbar drain was removed due to the absence of liquorrhea. The nasal cavity was tamponed with a gauze turunda. Since the volume of blood loss was 2,000 mL, hemotransfusion was performed intraoperatively.
The patient was mobilized 1 day after the surgery. The nasal tamponade was removed on the 5th postoperative day. Nasal breathing was completely restored. Control MRI showed no residual tumor (Figure 6).
Histological analysis revealed that the tumor was represented by hypercellular areas of chondrocytes with a mild cellular polymorphism, which consisted in a slight enlargement of these cells compared to typical chondrocytes, moreover, two nucleoli were observed in some of these cells (Figure 7). The tumor stroma was represented by a chondroid matrix with slight signs of myxoid degeneration.
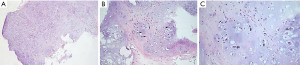
Nevertheless, since there were no pronounced signs of cellular, nuclear or structural atypia, foci/fields of necrosis, high mitotic activity in the tumor, it could not be classified as grade 2–3.
In this case, the diagnosis of G1 chondrosarcoma was established on the basis of the morphological characteristics described above, as well as taking into account the destructive nature of tumor growth.
The patient was discharged on the 7th postoperative day (Figure 8). Taking into account the results of histological report of the tumor and the impossibility of R0 resection considering the tumor location, the patient was referred for a proton beam therapy of the area of the removed tumor.
Discussion
In this article, we reported the case of a young male patient with a chondrosarcoma of the nasal cavity extended to the base of the skull. We performed a radical removal of the tumor via an endoscopic endonasal approach. The patient was referred to proton beam therapy of the bed of the removed tumor afterwards.
Chondrosarcomas usually arise from precursors of chondrocytes or osteocytes, but can also arise from primitive mesenchymal cells (1). Trauma, exposure to radiation, asbestos, beryllium, teflon, aluminum, iron, or radioactive isotopes are hypothesized etiological factors, however the cause of chondrosarcoma is still unknown (1).
Between 12% and 38% of chondrosarcomas develop secondary to previous conditions such as single or multiple exostoses, Maffucci syndrome, Paget’s disease, Ollier disease, and fibrous dysplasia. Most chondrosarcomas are sporadic lesions without any known causative factor (1,10). The average age of onset of the disease ranges from 40 to 50 years. Men are more often affected (1,11).
Chondrosarcoma is a slow-growing tumor (12). Symptoms of chondrosarcoma depend on its location and invasion or compression of intracranial structures. Chondrosarcoma usually does not metastasize until a very late stage but has a high tendency to local recurrence (3,4,13). If distant metastases develop, they first appear in the lungs (14,15).
The main method of treatment is a microsurgical resection, usually performed via open approaches (5,6). In the last decade, endoscopic endonasal approaches have been used to treat a variety of skull base tumors. However, only a few cases of endoscopic endonasal resection of intracranial chondrosarcomas have been reported (6,7,9,16).
The largest study described 30 patients with intracranial chondrosarcomas undergoing endoscopic endonasal resection (6). Radical resection was achieved in 19 patients (63.3%). Complications were observed in 10 (33.3%) patients, including 6 cases of liquorrhea (20%), 3 cases of persistent increase in neurological deficit (10%), 2 cases each of transient neurological disorders, meningitis, deep vein thrombosis, diabetes insipidus and one case each of sepsis and transient renal insufficiency. Most often, the radical resection of tumor could not be achieved when it extended to the cavernous sinus, the pyramid of the temporal bone, as well as between the foramen lacerum and the internal auditory canal. Resection of intracranial chondrosarcomas with staged and combined surgeries may be considered for larger tumors, especially tumors extending towards the clivus, foramen magnum, parapharyngeal space, and upper cervical spine (6).
In the case of an extensive postoperative defect and cerebrospinal fluid leakage, plastic surgery is performed using a muco-periosteal flap, a wide fascia fragment, and various auto-materials. The case of performing plastic surgery of the base of the anterior cranial fossa with a skin-aponeurotic flap on the feeding leg is also described (17).
Most authors recommend adjuvant postoperative radiotherapy because historical data on outcomes demonstrate a high local recurrence rate (1,4,6). Radiation therapy as the main treatment modality is usually prescribed for patients with large inoperable tumors (1,18). The recommended dose for radiotherapy is 60 to 70 Gy in 30–35 fractions (18). There is also an opinion that the radiosensitivity of intracranial chondrosarcomas may increase in patients with more than subtotal tumor resection (19). Reports of radiation toxicity ranged from 2% to 22% among various modalities of radiotherapy (6).
Five-year relapse-free survival rates in subtypes I, II and III, mesenchymal and myxoid subtypes are 15%, 16%, 33%, 63% and 16%, respectively, and 5-year survival rates are 90–95%, 81–90%, 43–75%, 46% and 94%, respectively (3,4). Oghalai et al. found that factors associated with an increased risk of tumor recurrence were younger age, large original tumor size, a residual tumor on postoperative imaging, and lack of postoperative radiotherapy (20).
Conclusions
Intracranial chondrosarcoma is a rare disease, the main treatment for which is a surgical removal. The maximal radicality and the creation of conditions for repeated surgical interventions are extremely important aspects of the surgery considering frequent local recurrence and relative radioresistance. An endoscopic endonasal approach is easily tolerated by the patient and facilitates minimally traumatic removal of chondrosarcomas with preservation of normal anatomy, thus creating comfortable conditions for repeated surgeries. However, we will be able to discover limitations of endoscopic surgeries and determine the strict indications for this method only after accumulation of sufficient experience. Adjuvant radiotherapy allows to reduce the frequency of local tumor recurrence.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-34/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-34/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-34/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sahu PK, Goyal L, Bothra J, et al. Chondrosarcoma of Nasal Cavity: a Rare Entity. Indian J Surg Oncol 2020;11:288-92. [Crossref] [PubMed]
- Korten AG, ter Berg HJ, Spincemaille GH, et al. Intracranial chondrosarcoma: review of the literature and report of 15 cases. J Neurol Neurosurg Psychiatry 1998;65:88-92. [Crossref] [PubMed]
- Murakami Y, Jinguji S, Kishida Y, et al. Multiple Surgical Treatments for Repeated Recurrence of Skull Base Mesenchymal Chondrosarcoma. NMC Case Rep J 2018;5:99-103. [Crossref] [PubMed]
- Bloch OG, Jian BJ, Yang I, et al. A systematic review of intracranial chondrosarcoma and survival. J Clin Neurosci 2009;16:1547-51. [Crossref] [PubMed]
- Almefty K, Pravdenkova S, Colli BO, et al. Chordoma and chondrosarcoma: similar, but quite different, skull base tumors. Cancer 2007;110:2457-67. [Crossref] [PubMed]
- Vaz-Guimaraes F, Fernandez-Miranda JC, Koutourousiou M, et al. Endoscopic Endonasal Surgery for Cranial Base Chondrosarcomas. Oper Neurosurg (Hagerstown) 2017;13:421-34. [Crossref] [PubMed]
- Frank G, Sciarretta V, Calbucci F, et al. The endoscopic transnasal transsphenoidal approach for the treatment of cranial base chordomas and chondrosarcomas. Neurosurgery 2006;59:ONS50-7; discussion ONS50-7. [PubMed]
- Moussazadeh N, Kulwin C, Anand VK, et al. Endoscopic endonasal resection of skull base chondrosarcomas: technique and early results. J Neurosurg 2015;122:735-42. [Crossref] [PubMed]
- Ditzel Filho LF, Prevedello DM, Dolci RL, et al. The Endoscopic Endonasal Approach for Removal of Petroclival Chondrosarcomas. Neurosurg Clin N Am 2015;26:453-62. Erratum in: Neurosurg Clin N Am 2015 Oct;26(4):xi. Kassam, Amin [Added]. [Crossref] [PubMed]
- Henry L. Surgical pathology of the head and neck, L. Barnes (Ed.). Inc., New York and Basel, Marcel Dekker Inc., New York and Basel, 1985. No. of pages: xxiv + 1896 (two volumes). Price: $300. ISBN: 0 8247 7216 4,08247.72695. J Pathol 1986;149:157-8.
- Bulut F, Kizilay A, Aydin NE. Chondrosarcoma of the nasal septum: a case report. Kulak Burun Bogaz Ihtis Derg 2004;12:39-41. [PubMed]
- Weber AL, Brown EW, Hug EB, et al. Cartilaginous tumors and chordomas of the cranial base. Otolaryngol Clin North Am 1995;28:453-71. [Crossref] [PubMed]
- Kim JH, Jung HH, Chang JH, et al. Gamma Knife surgery for intracranial chordoma and chondrosarcoma: radiosurgical perspectives and treatment outcomes. J Neurosurg 2014;121:188-97. [Crossref] [PubMed]
- Lee SY, Lim YC, Song MH, et al. Chondrosarcoma of the head and neck. Yonsei Med J 2005;46:228-32. [Crossref] [PubMed]
- Hong P, Taylor SM, Trites JR, et al. Chondrosarcoma of the head and neck: report of 11 cases and literature review. J Otolaryngol Head Neck Surg 2009;38:279-85. [PubMed]
- Zhang Q, Kong F, Yan B, et al. Endoscopic endonasal surgery for clival chordoma and chondrosarcoma. ORL J Otorhinolaryngol Relat Spec 2008;70:124-9. [Crossref] [PubMed]
- Ciporen J, Lucke-Wold B, Gillham H, et al. Paramedian Forehead Flap for Repair of Refractory High-Flow Anterior Skull Base CSF Leak. Turk Neurosurg 2017; Epub ahead of print. [Crossref] [PubMed]
- Burkey BB, Hoffman HT, Baker SR, et al. Chondrosarcoma of the head and neck. Laryngoscope 1990;100:1301-5. [Crossref] [PubMed]
- Saito Y, Takemura S, Sakurada K, et al. Intracranial extraskeletal mesenchymal chondrosarcoma arising from falx: a case report and literature review. No Shinkei Geka 2010;38:441-8. [PubMed]
- Oghalai JS, Buxbaum JL, Jackler RK, et al. Skull base chondrosarcoma originating from the petroclival junction. Otol Neurotol 2005;26:1052-60. [Crossref] [PubMed]
Cite this article as: Mikhailov NI, Zaitsev AM, Rerberg AG, Kisarev SA, Khokhrikov GI, Lazarev AE, Kirsanova ON, Kobyletskaya TM, Bezbabicheva TS, Krivobokova AV, Shegay PV, Kaprin AD. Endoscopic endonasal removal of nasal cavity chondrosarcoma extending to the base of the skull: a case report. Precis Cancer Med 2023;6:9.