
Sporadic mesenteric desmoid-type fibromatosis with “double-hit” T41A and S45P beta-catenin mutation profile: a case report of an extremely rare event—clinically relevant or much ado about nothing?
Introduction
Desmoid-type fibromatosis (DTF) is a rare non metastasizing clonal fibroblastic proliferation, arising from fascial or deep musculoaponeurotic structures (1,2). It is characterized by local invasiveness and unrelenting recurrences with recurrence rates ranging from 19% to 38% (1-4) and overall unpredictable biology. It primarily affects young adults, especially females with a predilection for the extremities, trunk, and intestinal mesenteries (2). While most desmoid tumors (approximately 85%) are sporadic, some occur as part of familial syndromes such as familial adenomatous polyposis (FAP), caused by germ-line mutations in adenomatous polyposis coli (APC) gene (5). Sporadic desmoid tumors are characterized by mutations in the gene-encoding β-catenin, CTNNB1. β-catenin is a cadherin-binding protein involved in cell-cell adhesion and a transcriptional activator of genes such as c-MYC that promote cellular proliferation and survival. The APC complex marks β-catenin for proteasomal degradation by sequentially phosphorylating four critical amino acids (serine 45, 37, and 33 and threonine 41), all encoded by exon 3 of the β-catenin gene where the majority of CTNNB1 mutations occur (6). Three major hotspot mutations within the CTNNB1 gene have been identified in sporadic cases of desmoid tumors: two occurring at serine 45 (S45F and S45P) and one at threonine 41 (T41A) (6). For the most part, these mutations appear to be mutually exclusive, though double-hit mutations have only been rarely reported.
Recent studies appear to suggest that CTNNB1-mutated desmoid tumors have a significantly worse recurrence-free survival than wild-type tumors and S45F mutated tumors have been shown to be more aggressive in some series (7-9). The therapeutic consequence of these specific mutations is not entirely clear. Given that desmoid tumors vary considerably in their clinical course and subsequent treatment strategies, there is an increasing trend towards genotyping these tumors with a view to identifying potential prognostic and therapeutic markers. Herein, we report an exceptionally rare case of mesenteric desmoid type fibromatosis harboring dual T41A and S45P mutations in exon 3 of the CTNNB1 gene. We present the following case in accordance with the CARE reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-2/rc).
Case presentation
A 74-year-old female with a history of gastric bypass and cholecystectomy presented with sharp, stabbing abdominal pain of sudden onset. A computed tomography (CT) scan (Figure 1A,1B) showed a large pelvic mass concerning for an adnexal neoplasm closely abutting the left common iliac artery. Intraoperatively, the mass appeared to be arising from the mesentery of the small bowel near the prior Roux-en-Y bypass (performed 8 years ago for morbid obesity). She underwent an exploratory laparotomy, adhesiolysis, bilateral salpingo-oophorectomy, small bowel resection, Roux-en-Y revision, and splenectomy.
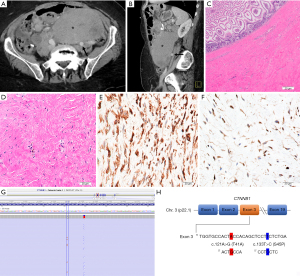
Gross examination revealed a 21.0 cm × 11.0 cm × 7.5 cm mass located on the mesenteric aspect of the small bowel. Sectioning of the tumor revealed a pale, tan-gray, diffusely fibrotic, homogenous cut surface devoid of any hemorrhage or necrosis. The mass grossly involved the muscularis of the small bowel. However, the mucosa remained uninvolved. Histopathologic examination demonstrated a banal pauci-cellular spindled cell neoplasm with variable collagenization and focal myxoid changes infiltrating the mesentery and small intestinal muscularis propria. The nuclei of the cells contained tiny nucleoli with no atypia. There was no tumor necrosis or appreciable increased mitotic activity. By immunohistochemistry, the lesional cells were variably positive for smooth muscle actin, focally positive for desmin and beta-catenin and negative for mdm2, DOG1, CD117, S100, MUC4 and CD34. The quantitative ki-67 proliferative index was estimated at <1% (Figure 1C-1F). Taken together, the overall features were most consistent with mesenteric DTF. To further confirm the diagnosis, CTNNB1 mutation analysis, performed by next generation sequencing identified the following mutations (Figure 1G,1H): DNA change c.121A>G resulting in an amino acid change p.T41A (Thr41Ala) as well as DNA change c.133T>C resulting in an amino acid change p.S45P (Ser45Pro). Both mutations occurred within the CTNNB1 gene confirming the extremely rare “double-hit” mutation profile. The patient did not receive any further systemic therapy after the initial surgical resection and is currently 18-months post-surgery with no evidence of recurrent disease. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Recent studies have shown CTNNB1-mutated desmoid tumors to have a significantly worse recurrence-free survival than wild-type tumors (7,8). Furthermore, among the CTNNB1 mutated tumors, those harboring the S45F mutation appear to behave more aggressively (6,10). Emerging data from recent large clinical studies and molecular profiling seems to indicate significant heterogeneity in tumor biology among the different types of DTF. Specifically, desmoid tumors with CTNNB1 T41A gene mutation are known to be associated with a better 5-year recurrence-free survival compared to those with S45F mutation, and desmoid tumors within the abdomen behave less aggressively than those in extra-abdominal sites (7,11,12). Indeed, the largest prospective outcome data on DTF from the French Sarcoma Group, suggests that anatomic location is the single most important determinant of event-free survival (13).
The optimal treatment of DTF remains a challenge with unacceptably high recurrence rates after various multi-modality approaches including active surveillance (watchful waiting), surgery, chemoradiation, anti-estrogenic, non-steroidal anti-inflammatory agents, tyrosine kinase inhibitors, and different molecular targeted therapies. The current treatment recommendation is to personalize therapy based on the consideration of several factors including tumor site, size, age, CTNNB1 mutation status, and coexisting morbidities. Precedent literature on the comprehensive molecular analysis of DTF by Mullen et al. uncovered a subset of sporadic DTF which harbor double mutations in exon 3 of CTNNB1 involving T41A and S45P accounting for approximately 1.9% of total cases within the series. The clinical characteristics of the rare double hit mutation profile was not further elaborated on in their series (11). Our index patient was treated with surgical resection alone with no systemic therapy and so far, has remained free of recurrence, 18 months after primary surgical treatment. So far, only double mutation profiles involving the T41A and S45P (considered favorable types) have been reported. Based on our anecdotal observation from the index case, one might be tempted to speculate that the desmoid tumors harboring double T41A and S45P beta-catenin mutations may not significantly alter treatment options or impact clinical outcome. Nonetheless, the clinical implications and therapeutic significance of this occurrence remains to be determined.
The role of tumor immune microenvironment in desmoid tumors has been previously described (14). Notably, T41A- and S45F-mutated desmoid tumors are known to have a differential expression of inflammation-related genes, compared to wild type DTF. Specifically, T41A-mutated cases, generally associated with better prognosis, have overexpression of anti-inflammatory/antitumor markers, such as CREB1, MAPK3, HMGN1, MKNK1 and IRF1, as well as lower levels of proinflammatory markers like TGF-β3 and TGF-β2. This peculiar inflammatory setting augments the role of beta-catenin signaling activation, thereby causing T-cell exclusion and immune evasion (evidenced by low tumor infiltrating lymphocytes and tumor associated macrophages and a low or absent expression of PD-1 and PD-L1) (14-17). Even though there is no published data on the pathways involved in “double-hit” CTNNB1 mutations in sporadic desmoids, similar inflammatory pathways might play a role. Furthermore, epigenetic alterations, such as aberrant DNA methylation, may also play a role (12,18). Given that oncogenes regulate the inflammatory milieu in tumors, it is speculated that by activating specific pro- and anti-inflammatory mediators, the “double-hit” CTNNB1 mutations might produce a unique inflammatory milieu. Whether this presumed “inflammation signature” results in a subsequent impact on clinical behavior remains to be seen.
The clinical course for DTF is unpredictable, ranging from spontaneous regression to locally aggressive behavior. However, to date, there are no molecular determinants that reliably and consistently identify tumors with a high risk for recurrence. While mutations in the gene-encoding β-catenin are frequent in sporadic desmoid tumors, it remains uncertain if the mutation status solely predicts clinical outcome (7,11,12). Furthermore, the question remains if patients with specific mutation profiles should be treated differently. Given the extremely rare occurrence of “double-hit” CTNNB1 mutations in sporadic DTF, there is currently no evidence, for now, that this entity behaves differently from tumors bearing either mutation types or if an aggressive (or different) therapeutic approach is warranted.
In summary, we presented a case of mesenteric sporadic desmoid fibromatosis in a 70-year-old female with “double-hit” CTNNB1 mutation at exon 3 involving both T41A and S45P, treated with surgical resection only. The limited data from precedent literature and the index case on this extremely rare subset of desmoid fibromatosis is insufficient to predict clinical outcomes. However, the information gleaned from the index cases suggests that a “double-hit” T41A and S45P beta-catenin mutation does not indicate aggressive clinical behavior compared to those with either T41A or S45P profiles. Consequently, there is a growing need to routinely perform CTNNB1 genotyping on desmoid tumors using sensitive platforms like next generation sequencing. Future research involving multicenter prospective patient accrual and integrated genomic and molecular characterization of sporadic desmoid fibromatosis would enable the elucidation of the underlying biology of these tumors and development of robust prognostic models and new personalized treatment strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-2/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-22-2/coif). OHI serves as an unpaid editorial board member of Precision Cancer Medicine from July 2020 to July 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leibel SA, Wara WM, Hill DR, et al. Desmoid tumors: local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys 1983;9:1167-71. [Crossref] [PubMed]
- Lev D, Kotilingam D, Wei C, et al. Optimizing treatment of desmoid tumors. J Clin Oncol 2007;25:1785-91. [Crossref] [PubMed]
- McKinnon JG, Neifeld JP, Kay S, et al. Management of desmoid tumors. Surg Gynecol Obstet 1989;169:104-6. [PubMed]
- Acker JC, Bossen EH, Halperin EC. The management of desmoid tumors. Int J Radiat Oncol Biol Phys 1993;26:851-8. [Crossref] [PubMed]
- Eccles DM, van der Luijt R, Breukel C, et al. Hereditary desmoid disease due to a frameshift mutation at codon 1924 of the APC gene. Am J Hum Genet 1996;59:1193-201. [PubMed]
- Alman BA, Li C, Pajerski ME, et al. Increased beta-catenin protein and somatic APC mutations in sporadic aggressive fibromatoses (desmoid tumors). Am J Pathol 1997;151:329-34. [PubMed]
- Lazar AJ, Tuvin D, Hajibashi S, et al. Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 2008;173:1518-27. [Crossref] [PubMed]
- Dômont J, Salas S, Lacroix L, et al. High frequency of beta-catenin heterozygous mutations in extra-abdominal fibromatosis: a potential molecular tool for disease management. Br J Cancer 2010;102:1032-6. [Crossref] [PubMed]
- Tejpar S, Nollet F, Li C, et al. Predominance of beta-catenin mutations and beta-catenin dysregulation in sporadic aggressive fibromatosis (desmoid tumor). Oncogene 1999;18:6615-20. [Crossref] [PubMed]
- Braggio D, Zewdu A, Londhe P, et al. β-catenin S45F mutation results in apoptotic resistance. Oncogene 2020;39:5589-600. [Crossref] [PubMed]
- Mullen JT, DeLaney TF, Rosenberg AE, et al. β-Catenin mutation status and outcomes in sporadic desmoid tumors. Oncologist 2013;18:1043-9. [Crossref] [PubMed]
- Timbergen MJM, Boers R, Vriends ALM, et al. Differentially Methylated Regions in Desmoid-Type Fibromatosis: A Comparison Between CTNNB1 S45F and T41A Tumors. Front Oncol 2020;10:565031. [Crossref] [PubMed]
- Penel N, Le Cesne A, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 2017;83:125-31. [Crossref] [PubMed]
- Colombo C, Miceli R, Lazar AJ, et al. CTNNB1 45F mutation is a molecular prognosticator of increased postoperative primary desmoid tumor recurrence: an independent, multicenter validation study. Cancer 2013;119:3696-702. [Crossref] [PubMed]
- Colombo C, Belfiore A, Paielli N, et al. β-Catenin in desmoid-type fibromatosis: deep insights into the role of T41A and S45F mutations on protein structure and gene expression. Mol Oncol 2017;11:1495-507. [Crossref] [PubMed]
- Dibra D, Mishra L, Li S. Molecular mechanisms of oncogene-induced inflammation and inflammation-sustained oncogene activation in gastrointestinal tumors: an under-appreciated symbiotic relationship. Biochim Biophys Acta 2014;1846:152-60. [PubMed]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231-5. [Crossref] [PubMed]
- Song J, Du Z, Ravasz M, et al. A Protein Interaction between β-Catenin and Dnmt1 Regulates Wnt Signaling and DNA Methylation in Colorectal Cancer Cells. Mol Cancer Res 2015;13:969-81. [Crossref] [PubMed]
Cite this article as: Shafi S, Patton A, Rogers A, Grignol V, Oghumu S, Iwenofu OH. Sporadic mesenteric desmoid-type fibromatosis with “double-hit” T41A and S45P beta-catenin mutation profile: a case report of an extremely rare event—clinically relevant or much ado about nothing? Precis Cancer Med 2022;5:28.

