
A narrative review of epidemiology and prevention of lung cancer: sex/gender differences?
Introduction
The model of tobacco smoking epidemic over time primarily explains the trends of smoking related morbidity and mortality in different populations or groups of populations (1,2). The smoking trend among women seems to follow the same pattern as that among men, with a lag of nearly a century (1). Whenever the characteristics of women who smoke become equivalent to those of men, the relative risk of death from smoking-related diseases, including lung cancer, is for females at least similar to that of males (2), which means that at least one-half of all women who smoke cigarette will eventually be killed by their habit (3).
However, the amount of tobacco smoking exposure does not fully explain the natural history of lung cancer: other environmental risk factors and different individual susceptibility play a role, even if their interaction is not completely understood (4,5).
Overall, the combined effect of “sex” and “gender” affects the state of health, and differences in lung cancer between male and female may result from the interaction of sex-dependent genetic factors and gender-dependent socio-cultural differences during life (6).
These interactions may affect many different aspects like the susceptibility, the disease progression, the relationship with the specialist and with pharmacological and non-pharmacological therapies throughout the life span. For this reason, sex and gender should be taken into account during research activities and clinical care to better tailor prevention strategies, diagnosis, and therapies (7).
The aim of this paper is to summarize sex/gender related differences in lung cancer by describing the epidemiological trend of the disease, molecular epidemiology findings, the burden of risk factors, the crucial role of prevention, mainly smoking cessation, and the possible sex/gender-related characteristics of preventive interventions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-54/rc).
Methods
This paper is divided into four main topics, describing the epidemiological trend of the disease, molecular epidemiology findings, the burden of risk factors, and the prevention strategies with a focus on smoking cessation. As regards data sources, PubMed Central and official websites of International and European Agencies and Societies were used. Recent evidence about potential differences between males and females in the lung cancer epidemiology (prevalence, temporal and spatial trend, biomarkers), risk factors (tobacco smoking and air pollution) and prevention was selected. The study was limited to published original manuscripts/reviews/reports in the English language from 1990 to 2021 (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 1 August 2021 to 1 March 2022 |
| Databases and other sources searched | PubMed Central and official websites of International and European Agencies and Societies |
| Search terms used | Search terms: Lung cancer AND (prevalence, temporal trend, molecular epidemiology, biomarkers, environmental tobacco smoke, passive smoke, outdoor air pollution, indoor air pollution, smoking habits, smoking cessation) AND (sex or gender or female or women) AND (English, from 1990/1/1–2021/12/31) |
| Timeframe | From January 1, 1990 to December 31, 2021 |
| Inclusion and exclusion criteria | Inclusion and exclusion criteria: (I) articles languages: English; (II) article types: original manuscripts, reviews, reports |
| Selection process | The authors conducted independently the selection based on the specific issue to be addressed |
Geographical and temporal trend in lung cancer
Geographical trend
A publication based on the International Agency for Research on Cancer (IARC) GLOBOCAN 2020 data, estimated 19.3 million new cancer cases and 10.0 million cancer deaths in 2020 worldwide (8). Overall, female breast cancer and lung cancer were the most commonly diagnosed cancer (11.7% and 11.4% of the total cases, respectively). Lung cancer remained the leading cause of cancer death (18.0% of the total cancer deaths).
GLOBOCAN 2020 data showed a different distribution of cases and deaths for lung cancer in males and females: the first rank for frequency (14.3%) and for death (21.5%) among males; the third rank for frequency (8.4%) and the second rank for death (13.7%) among females (8).
However, this distribution relies heavily on the socio-economic and life style factors, like smoking habits (8). In industrialized nations, as a result of smoking habits changes, lung cancer rates have increased or stabilized in women and a decreased in men. In developing countries, smoking habits as well as other risks factors such as indoor and outdoor environmental pollution can vary widely, consequently influencing lung cancer rates.
The incidence rates (new cases) for lung cancer varied from 2.8% in Western Africa to 51.6% in Micronesia/Polynesia in males, with values larger than 35% in Europe (East, South and West: 49.0%, 43.1%, 41.7%, respectively), Asia (East and West: 48.1%, 41.7%, respectively) and Northern America (35.7%) in 2020. In females, the incidence rates for lung cancer varied from 1.8% in Western Africa to 30.1% in Northern America, with values larger than 20% in Europe (West and North: 25.0%, 26.8%, respectively), in Micronesia/Polynesia (22.9%), in Australia/New Zealand (22.7%) and in Eastern Asia (22.1%) (8) in 2020. It is of note that, in China, the lung cancer incidence rates among females who show a low smoking prevalence, were similar to those of Western European females, who show a high smoking prevalence. This finding may be due to an increased exposure to smoke from burning of charcoal in women living in China (8).
Europe represents a special situation, including only 9% of the world population, but 25% of the global cancer burden. In 2018 about 4 million new cases of cancer and 2 million deaths from cancer were estimated in Europe (9). Lung cancer was the second most frequent cancer (15.1%) and the leading cause of cancer death (24.8%) among European males; the third (8.5%) most frequent cancer and the second cause of cancer death (14.2%) among European females (9); these values were in line with those reported in the GLOBOCAN 2020 data, worldwide (8). In EU-28, lung cancer has become the leading cause of death from cancer in both males (24.5%) and females (16.4%) in 2018 (9).
Temporal trend
The temporal trends in lung cancer incidence and mortality also reflect the trends in prevalence of tobacco smoking in the country (1,2). Lung cancer incidence rates peak 30–40 years after the peak of smoking prevalence (1). A US study about the 50-year trends in smoking-related mortality showed that the risk of death from lung cancer in smoking females is continuing to increase (2.73, 12.65, and 25.66 respectively in the 1960s, 1980s, 2000s cohort), and today the difference between males and females is narrowing. In relative terms, today the risk is similar in both males and females, increased about 25 times than those who don’t smoke (2). As reported by the Authors, this data confirmed the prediction by Richard Peto that “if women smoke like men, they die like men” (10).
Considering the period from 1950 to 2014, lung cancer death rates among females have stabilized or declined where smoking habits in women started early, to decrease later (e.g., Hong Kong, the United Kingdom, and Australia), but they have continued to rise in the regions where this habit in women began later (e.g., Europe and Latin America). In many of these countries, mortality rates in young females had begun to decline in recent years with the entry into force of tobacco control measures (1).
The prevalence of tobacco smoking is decreasing worldwide, except for the World Health Organization (WHO) African and East Mediterranean regions, where the trends appear to be flat. WHO estimated that the prevalence of tobacco smoking by people aged ≥15 years was 20.2% in 2015 (34.1% in men and 6.4% in women), with a projected prevalence of 17.3% in 2025 (30.0% in men and 4.7% in women) (11).
In the US, a projection of the reduction in tobacco use and lung cancer mortality due to existing tobacco control efforts was estimated from 2015 to 2065: age-adjusted lung cancer mortality is projected to drop 79% for both males and females combined, with a greater reduction in males (83% males vs. 73% females). The projected age-adjusted lung cancer mortality rate for females and males is expected to become nearly the same by 2045 (20.0 per 100,000) (12).
In Europe, all cancer mortality has been declining since the late 1980s in males, mainly driven by a steep reduction in mortality rates from lung and stomach cancers. The same trend emerged in European females, except for lung cancer and breast cancer (13). In particular, lung cancer, for the period 1994 and 2012, showed a nearly linear decrease in mortality rates from 76.71 to 56.84 per 100,000 in males and an increasing trend from 15.00 to 20.50 per 100,000 in females, reducing the sex gap during the study period from 5.1 (male/female ratio) in 1994 to 2.8 in 2012 (14). In 2020, the highest EU predicted mortality rates in both sexes were for lung cancer, but, showing a decline by 9.2% in males and an increase by 6.0% in females between 2015 and 2020 (13).
Conversely, in China, the standardized mortality rate of lung cancer has constantly increased from 1991 to 2013 (from a standardized mortality rate of 14.47 to 26.89 per 100,000) in both males and females, and it was expected to reach 33.49 per 100,000 in 2018 (15). The standardized mortality rate of lung cancer was higher in males with respect to females, in urban areas than in rural areas and in people living in northeast China provinces and the coastal provinces in eastern China than those living in the centre or western Chinese provinces (15).
Sex/gender-differences in molecular epidemiology of lung cancer
There are sex differences in therapeutic response and toxicity for various types of cancer, including lung cancer. As regards lung cancer chemotherapy, females show higher toxicity and response rates, and longer post-treatment survival suggesting an interaction between estrogen levels and chemotherapy prescriptions optimizing efficacy of treatment (7).
Recent studies have shown a higher risk of developing the main histologic lung cancer types in women than men who smoke regardless of baseline level of exposure (16), smoking history, or body size (17). Therefore, gender differences in susceptibility to tobacco carcinogens could be supposed (17). Since the DNA adducts excess in smokers, their use as biomarkers predictive of lung cancer is suggested (18). In comparison to males who smoke, females who smoke show higher levels of polycyclic aromatic hydrocarbon-DNA adducts (19). These outcomes could be due to a synergistic interaction between estrogens and tobacco compounds through the induction of CYP1B1, an enzyme responsible for estrogenic metabolism, which leads to enhanced reactive oxygen species formation and carcinogenesis (20). Moreover, females present decreased DNA repair capacity and more common p53 mutations, both in people who smoke and in those who don’t smoke. Females present also increased capacity of CYP1A1 gene to convert tobacco carcinogens to activated metabolites (21).
Sex hormones like estrogen have been supposed to contribute to lung cancer development and progression (17). In the Jiangsu Four Cancers (JFC) study, later menopause and increased number of ovulatory cycles were found to be associated with increased risk of lung cancer, on the contrary a higher parity and gravidity were associated with reduced risk of lung cancer (22). Furthermore, there are data about the potential decreased risk in females undergoing long term antiestrogen therapy (21). Further, estrogen receptors α (ERα) expression, higher in stage I lung adenocarcinoma cells of females than of males, was suggested an independent predictor of recurrence in pT1a stage (size ≤2 cm) lung cancer (23).
There are inconsistent data about hormone replacement therapy (HRT) and lung cancer. Although treatment with estrogen plus progestin in postmenopausal women does not appear to increase the incidence of lung cancer, it was found to increase mortality from lung cancer (24). These findings should be considered when evaluating combined hormone therapy risk-benefit for women, especially those with a high risk of lung cancer.
Risk factors
Several factors that increase individual risk of developing lung cancer have been identified, most are associated with socioeconomic development (6). The most common include lifestyle (e.g., dietary habits, heating and cooking practices), environmental and occupational hazards. Area of residence, sex, ethnicity and genetic predisposition, alone or in combination, can however affect the role of these factors (4). Gender-related factors may also influence lung cancer risk (5,6,21,22,25).
Even if smoking is the most important risk factor of lung cancer, the incidence among women who don’t smoke is globally increasing, so it becomes important to investigate the influence of the other factors: environmental tobacco smoke (ETS), air pollution both outdoor and indoor, occupational and nonoccupational exposure to hazardous chemicals and radon exposure from soil and building materials (1,5,26-30). Dietary habits, alcohol consumption, marijuana smoking, estrogen, infections with human papilloma virus (HPV), human immunodeficiency virus (HIV), and Epstein-Barr virus are suggested to be linked with lung cancer but there are not sufficient evidences to confirm their relation (26,31,32).
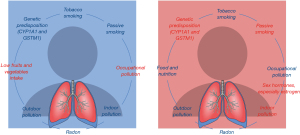
A summary of the evidences about the influence of risk factors for lung cancer by gender/sex was reported in Table 2 and Figure 1. Due to their relevance, this paper summarizes the role of tobacco smoking and air pollution on lung cancer, especially in females.
Table 2
| Risk factors | Influence | References |
|---|---|---|
| Lifestyle | ||
| Tobacco smoking | = | (6,22,26,28,33,34) |
| Low fruits and vegetables intake | M | (26,32) |
| Environment | ||
| Outdoor air pollution | – | (35-37) |
| Indoor air pollution | F | (26,28,38,39) |
| Passive smoking | F | (4,22,28,40) |
| Occupational pollution | M | (26,27) |
| Radon | – | (30) |
| Genetic | ||
| Sex hormones, especially estrogen | F | (5,6,22) |
| Genetic predisposition (CYP1A1 and GSTM1) | F | (21,25) |
M, higher risk in males; F, higher risk in females; –, no evidence of different risk in males and females; =, similar risk in males and females.
Active smoking
Geographic variation in lung cancer is primarily related to tobacco use (1). Very high attributable risks (about 90% for men and 60% for women) and odds ratios (23.6 for men who smoke and 7.8 for women who smoke) were reported for tobacco smoking (22,33), whereas more recently similar risks have been found in both sexes (6,34).
According to the Center for Disease Control and Prevention (CDC), cigarette smoking is the major cause of the disease but other tobacco products such as cigars or pipes increase the risk for lung cancer (41). Tobacco smoke is a toxic mix that contains numerous mutagens and carcinogens, such as polycyclic aromatic hydrocarbons and tobacco-specific nitrosamines, which are known to cause cancer in people and animals (42).
The relevance of tobacco smoking as the main attributable risk factor for lung cancer is also suggested by the decrease of lung cancer mortality observed over time after the decrease in its prevalence in males (43). On the contrary, there have been increasing trends in lung cancer incidence and mortality among females, probably due to the increasing prevalence of smoking, although females have better lung cancer survival than males globally (22).
In Japan, approximately 40 years after the start of the trend reversal in males, a decrease in the age-adjusted lung cancer mortality rate was observed, similar to what happened in the USA and UK (43). In Italy, the smoking prevalence decreased in men from 65.0% in 1957 to 23.9% in 2017 but in women it increased, over the same period of time, from 6.2% to 21.0% (reaching the highest value in 2008, 25.9%), as well as lung cancer incidence and mortality since the 1990s (44). The histological subtypes show a different distribution by sex: females who smoke are more likely to develop lung adenocarcinoma while male who smoke squamous cell carcinoma (44).
A recent study has also found a dose-dependent association between cumulative cigarette tar exposure and risk of lung cancer (especially squamous and small cell carcinoma), among Japanese who smoke. According to the authors, there is no level of smoking exposure which could be considered risk-free, since low-tar exposure also showed an association with an increase in lung cancer risk (45).
Environmental tobacco smoking
Women who do not smoke have often been exposed to high levels of environmental tobacco smoking (ETS or passive smoking or second-hand smoke), especially before the advent of indoor smoking bans (46,47). ETS has been shown associated with increased risk of lung cancer for all major histologic types (22). Second-hand smoke was classified as a carcinogen by the Environmental Protection Agency; it was reported that ETS exposure also leads to a dose-dependent risk of lung cancer (4,5). ETS is estimated to cause over 600,000 deaths worldwide, most of them in females (4): 21,400 deaths for lung cancer annually in people who do not smoke (4). Scientific evidences have reported an increase in lung cancer risk by 20% to 30% due to exposure to second-hand smoke (4). The IARC reported an increased lung cancer risk of 20% for women who do not smoke and who are exposed to second-hand smoke from their partners (40).
A recent meta-analysis has confirmed that the risk for lung cancer among people who do not smoke exposed to ETS compared with those not exposed was increased by 24% and this risk was dose-dependent, correlating with number of cigarettes smoked and duration of exposure. Furthermore, this association appears strongest in females (48). A US study reported that the relative risk of developing lung cancer was higher in non-smoking women with smoking husbands than in non-smoking men with smoking wives (RR 1.2 versus 1.1) (49).
Passive smoking and indoor pollution related lung cancer deaths are mainly spread in lower to middle-income countries, in particular in China. In fact, lung cancer rates are higher in women living in China than in several European countries, despite their lower smoking prevalence (1). In a prospective study of over 600,000 UK women who did not smoke, lung cancer incidence was significantly associated with non-white ethnicity, taller stature and asthma medications intake (50).
Outdoor pollution
According to a WHO report, 14% of lung cancers are attributable to outdoor air pollution and 17% to indoor air pollution (51). Indeed, over the past decades, much scientific evidence has been accumulated about the role of indoor and outdoor air pollution on lung cancer development (47). Outdoor particulate matter (PM) was associated with adenocarcinoma (44).
Exposure to outdoor air pollution is ubiquitous, affecting everyone, and has numerous serious adverse human health effects, including cancer. In June 2012, the IARC classified diesel engine exhaust as carcinogenic to humans (Group 1) (52), as well as, in 2016, the PM in outdoor air pollution (53), on the base of sufficient evidences that exposure is associated with an increased risk of lung cancer. Air pollution, and especially long-term exposure to fine PM, has been associated with increased lung cancer risk and mortality, regardless of cigarette smoking, in several epidemiologic studies (54).
A recent meta-analysis selecting study from North America, Europe and Asia showed a RR of 1.14 (95% CI: 1.08–1.21) in lung cancer incidence or mortality per 10 µg/m3 PM2.5. At the global population level, mean annual PM2.5 concentration of 46 µg/m3 in respect to the 2005 WHO air quality guidelines limit (10 µg/m3) leads to about 60% excess of lung cancer risk (55). In original studies even higher values of lung cancer risk were found: OR 1.30 (95% CI: 1.02–1.66) in highly polluted areas in Italy for PM10 (35) and a mortality rate ratio of 1.36 (95% CI: 1.23–1.50) in a nationwide Danish study for PM2.5 (56).
Significant adverse associations were reported between nitrogen dioxide (NO2) exposure, a marker of traffic-related air pollution, and lung cancer mortality in meta-analyses (RRs 1.04–1.05 per 10 µg/m3 NO2 increase) (36,57). In a recent Danish nationwide study, a mortality rate ratio of 1.17 (95% CI: 1.15–1.18) emerged (56).
In a US study on a cohort of postmenopausal females who didn’t smoke, no significant association of PM2.5 and NO2 exposure with lung cancer risk emerged. On the contrary, an increased risk was observed comparing individuals living within 50 meters from a primary limited access highway versus those living at a distance ≥200 meters (hazard ratio 5.23, 95% CI: 1.94–14.13). This result suggested the possibility of using residential proximity to major roadways as a proxy for carcinogenic exposures (58).
Additional researches in Asia and in highly polluted regions are needed, as well as researches taking into account the modification of outdoor air pollution associations by other individual or lifestyle factors (55).
Indeed, contrasting results emerged considering the possible joint effects of air pollution and cigarette smoking. Associations with PM2.5 were somewhat stronger in persons with smoking history (RR 1.44; 95% CI: 1.04–2.01) than in those who didn’t smoke (RR, 1.18; 95% CI: 1.00–1.39) (59); the association between PM10 exposure and lung cancer risk was lower and no longer significant after adjusting the statistical analyses for smoking habits (OR 1.30, 95% CI: 1.02–1.66 vs. OR 1.17, 95% CI: 0.88–1.55) (35). In an analysis of US Cancer Prevention Study II, evidence of interaction between ambient PM2.5 and cigarette smoking emerged, with higher risk for lung cancer mortality among subjects with both exposures. It was estimated that 14% of lung cancer deaths were attributable to the interaction between these two factors (60).
As regards NO2, in a meta-analysis of studies conducted in North America and Europe the same relative risk for lung cancer incidence or mortality was found after adjusting for smoking habits (RR 1.04, 95% CI: 1.01–1.08) (36); on the contrary, in another meta-analysis stratifying the studies between those without individual adjustment for body mass index and smoking and those with individual adjustment, significant results were confirmed for lung cancer mortality only in the first ones (HR 1.06, 95% CI: 1.03–1.10 vs. HR 1.02, 95% CI: 0.96–1.08) (57).
As regards sex difference in the effect of air pollution on lung cancer, there are no studies that specifically took into account this aspect. Nevertheless, some evidence suggested that health responses to air pollution may differ between females and males. For instance, an Italian study (61) performed in highly air polluted areas, close to coal-fired power stations that emitted high concentrations of heavy metals, reported a significant increase in lung cancer mortality in females but not in males.
Indoor pollution
Indoor pollutants resulting from domestic combustion of biomass fuel (mainly wood) and cooking oil fumes have been classified as carcinogenic or probable carcinogen to humans by the IARC (62); indoor emissions from household combustion of coal were classified as human carcinogen (group 1) (63).
The biomass combustion is a relevant source of indoor pollutants: fine particles, black carbon, dioxins, volatile organic compounds (VOCs), including mono- and polycyclic aromatic hydrocarbons (64). It may cause short and long-term health effects (64).
Indoor air pollution due to the use of solid fuels (wood, crop residues, dung, and coal) for heating and cooking is most common in approximately half of the world’s population especially in low- and middle-income countries in Africa and in eastern and southern Asia (1,4).
A meta-analysis including studies conducted in Asia, Mexico, Morocco, USA, Canada, and in seven European countries suggests that indoor coal and biomass combustion is associated with an increased risk of lung cancer (OR 1.82, 95% CI: 1.60–2.06 and OR 1.50, 95% CI: 1.17–1.94, respectively). Further, the risk of lung cancer due to solid fuel use (coal or biomass) was significantly greater in females (OR 1.81, 95% CI: 1.54–2.12) than in males (OR 1.16, 95% CI: 0.79–1.69). As regards the histological subtypes, the highest risk was found for squamous cell carcinoma (OR 3.58, 95% CI: 1.58–8.12), followed by adenocarcinoma (OR 2.33, 95% CI: 1.72–3.17) and unspecified cell type tumors (OR 1.57, 95% CI: 1.38–1.80) (38).
In another recent meta-analysis, cooking oil fume resulted a risk factor for lung cancer only in females, regardless of smoking status: OR 1.98 (95% CI: 1.54–2.54) among those who don’t smoke and 2.00 (95% CI: 1.46–2.74) among those who partly smoke. For cooking males, the pooled OR of lung cancer was 1.15 (95% CI: 0.71–1.87) (39).
A Chinese population-based case-control study reported a protective effect of good home ventilation on lung cancer, probably due to the reduction of exposure to indoor air pollutants. Ventilation may therefore represent a preventive measure for lung cancer, in addition to tobacco cessation (65).
Smoking cessation
Benefits
The primary preventive measure for lung cancer is “not-to-start smoking”, but the risk of lung cancer can be also significantly reduced with tobacco cessation, especially discontinuing smoking habit early in life. People with smoking history have a lower risk of lung cancer compared with those who smoke, and the risk declines with the number of years of smoking cessation. Smoking cessation brings benefits for people with or without smoking-related disease in both sexes (66).
Many studies confirmed the benefit of smoking cessation in increasing life expectancy and decreasing mortality. In a prospective study it has been seen that on average a British man who smoke dies 10 years younger than a man who does not smoke, while smoking cessation at age 50 reduces the risk of a half and at age 30 eliminates almost all risks (3). The “Million Women Study” has been analyzed the first generation of women (born around 1940) who smoked nearly like men: it found that among UK women who smoke, two-thirds of all deaths are caused by smoking. Stopping the smoking habit before age 40 avoids more than 90% of the excess mortality caused by continuing to smoke (67).
A study conducted in Italy was aimed to estimate the “life gain” in terms of additional number of years that a person who smoke can live stopping smoking. For both sexes the years of life gained with smoking cessation was directly proportional with the number of cigarettes smoked per day and the younger age of quitting smoke (greatest benefits before 35 years of age) (68). The life gain seems to be higher in men than in women: for example, a man who smokes a mean of 15 cigarettes per day and who quit at the mean age of 40 years, gains a 19% of life (i.e., 7 years gained over 36 years of additional life expectancy if continuing smoking), as compared to a woman who gains a 12% of life (i.e., 5 years gained over 41 years of additional life expectancy if continuing smoking) (68).
The benefits of smoking cessation have been demonstrated also in patients with cancer. A recent paper reported that patients with cancer and an active tobacco use who do not quit smoking have a poor prognosis, a worse quality of life, a higher risk of new primary cancer and an increased treatment-related toxicity (69). Moreover, smoking cessation can improve the therapeutic outcomes in patients with lung cancer, and the most effective method to help smoking cessation in these patients may be the intensive smoking counseling with the support of medication (70).
Sex/gender differences in successfully quitting smoking
Many studies have identified gender/sex differences in relation to quitting smoking successfully. Some data suggest that women are less likely to quit smoking habit than men, especially when trying to quit on their own. An Italian study engaging patients in a lung cancer screening program (ITALUNG) found that quitting smoking during the screening program was significantly associated to male/sex gender (71). On the other hand, data from smoking cessation services in UK do not support this evidence (72) and the large multicenter interventional EAGLES trial did not observe differences in men and women about success rate for smoking cessation (73).
The variation of gender/sex differences about smoking cessation has been analyzed in a review (74): data coming from efficacy and effectiveness trials supported the evidence that women may have more difficult in maintaining long-term abstinence while data from cross-sectional and observational studies were inconclusive (Table 3). This can be explained by the numerous bio-psycho-social variations in samples analyzed and by different timing and location of the studies (74).
Table 3
| Type of study | Total number of studies [sex/gender difference tests] | W < M | W > M | W = M |
|---|---|---|---|---|
| Efficacy trial | 37 [47] | 25 | 1 | 21 |
| Effectiveness trial | 77 [79] | 34 | 1 | 44 |
| Community-based interventions | 4 [4] | 2 | – | 2 |
| Prospective observation studies | 40 [46] | 10 | 5 | 31 |
| Cross-sectional observational studies | 32 [38] | 11 | 9 | 18 |
| OVERALL | 190 [214] | 82 | 16 | 116 |
W < M, higher rate of success in men than in women; W > M, higher rate of success in women than in men; W = M, no differences or inconclusive results [data from ref. (74)].
In a large population study, women seem to be more likely to quit smoking than men, considering subjects under 50 years, but the opposite occurs in those over 50 years; however, across all age groups there was no significant gender difference in smoking cessation rate (75). By opposing gender stereotypes, we agree with the authors of the mentioned study that women should not be considered less capable than men to quit smoking successfully.
Several hypotheses have been formulated to explain possible sex/gender differences in successful smoking cessation (Table 4). Different patterns have been identified in terms of smoking habits and personal characteristics. An important factor that may promote smoke cessation among women of childbearing age is pregnancy, that could explain why young women are more likely to quit smoking than men, as reported in some cross-sectional studies conducted in the USA, Canada and the UK (75). Data suggest that in a woman who give birth to one child the smoking cessation rate increases by 40% compared to a woman with no children and the rate still increases up to 120% in case of three or more children. Men who have children, regardless of the number, also reported a higher smoking cessation rate (25–30%) (76). Unfortunately, even if the number of women who quit smoking is higher during pregnancy compared to other times of their lives, two thirds of them have a relapse within one year from the delivery (77).
Table 4
| Cons | Pros |
|---|---|
| Women fear and are more susceptible to weight gain than men after smoking cessation. Women are more likely than men to use tobacco to control their weight | Pregnancy promotes smoking cessation among women to a greater degree than parenting do in men |
| Women show lower levels of self-efficacy and are more likely to identify with social barriers to cessation | More frequent use of coping strategies among women. Some women are able to self-control their tobacco use |
| Women show greater sensitivity to non-nicotine environmental cues (sight, smell, and sensations of smoking) | Women generally appear to be less nicotine-dependent than men showing lower sensitivity to nicotine itself and having higher rates of nicotine metabolism than men |
| Studies have shown a higher prevalence of depression and anxiety among women. Women are more likely to use tobacco to cope with negative feelings | Less consumption of other tobacco products than men |
| Women show more severe withdrawal symptoms and cigarette craving, especially related to luteal phase of menstrual cycle | Marital status appears to be more beneficial to women as they are more sensitive to social support in quitting smoking |
Other factors that seem to influence smoking cessation and relapse in females are: fear of weight gain, depression, severe cigarette craving, nicotine dependence, hormonal factors, need for social support and use of other tobacco products.
Among women, smoking cessation is typically associated with a weight gain greater than among men and therefore, when trying to quit smoking, women seem to fear weight gain more than men. However actual weight gain during cessation does not predict relapse to smoking (77).
Negative mood plays an important role in smoking behavior, especially among women who have a lifetime prevalence of depression and anxiety higher than in men. Some studies showed women’s greater propensity to use tobacco to deal with anxious or depressive moods, to relapse in stressful situations or to exhibit severe negative mood symptoms and cigarette craving after a few hours of abstinence from smoking (78). However other studies did not find such differences and the topic is still debated (78). Most of the evidence reports that women who smoke suffer more from nicotine withdrawal symptoms than men who smoke when trying to quit, but it has been less clear whether resumption of smoking produces greater relief from these symptoms in women (78).
A potentially important factor that may influence on withdrawal symptoms is menstrual cycle. Due to hormonal changes during the menstrual cycle, smoking desire and abstinence-related symptoms fluctuate across the menstrual cycle phases: luteal phase seems to be particularly associated with increased smoking habit (79).
Furthermore, tobacco dependence seems to be different between men and women; research suggests that women are generally less sensitive to nicotine itself and more sensitive to non-nicotine factors, compared to men. Thus, smoking behaviour of women seems to be less reinforced by nicotine and more by sensory effects of smoking and psychological factors (like social interaction and tension reduction) than that of men (71). In addition, some studies found that, compared to men, women have a higher perception of social exclusion as a risk of quitting smoking and show lower levels of self-efficacy in smoking cessation (80,81).
Despite these findings, women seem to use more frequently than men self-change strategies such as keeping busy, taking deep breaths, or concentrating on other things to overcome the urge to smoke (82). The more frequent use of these strategies to take control of smoking suggests that women can control their smoking permanently: it could explain why men are more likely to smoke heavily than women (82). Furthermore, women also have lower plasma cotinine concentrations, a metabolite of nicotine, than men regardless of body weight. This result can be partially attributed to estrogen. Indeed, a greater clearance of nicotine is found in women who take estrogen as hormone replacement or contraceptive therapy (83).
Moreover, it has been seen that marital status appears to be more beneficial to women as they are more sensitive to social support in quitting smoking (84).
Lastly, gender differences have also been found in the use of other tobacco products after quitting smoking. Women are less likely to switch to pipe, cigars, or smokeless tobacco after quitting cigarettes than men (77).
Sex/gender differences in smoking cessation treatment
Tobacco addiction is a chronic disease that often requires multiple interventions and attempts to treat. Fewer than 10% of people who smoke attempting to quit on their own are successful over the long term but success rate increases in those who smoke seeking professional help (85). Scientific evidence shows that behavioral support (brief advice and counseling) and pharmacotherapy are effective when used separately, however the combination of the two achieves the best results (86).
Many pharmacologic approaches are available for smoking cessation. The first line therapies are Nicotine Replacement Therapy (NRT) (including gum, transdermal patch, nasal spray, or vapour inhaler), bupropion and varenicline (86,87). A meta-analysis about gender differences in nicotine patch efficacy reported equal efficacy in both genders (88), while other analysis reported evidence of better results among men (89). From the results of the International Tobacco Control Four Country Survey, women were more likely to discontinue the use of NRT because of side effects but to benefit from NRT at long-term follow-up if they received the treatment in conjunction with psychological intervention (90).
A meta-analysis investigating gender differences in bupropion efficacy found similar results in both genders (91), although some studies showed less efficacy in women (92). Regarding varenicline, data form clinical trials demonstrated greater efficacy among women who smoke than among men who smoke for short and immediate-term outcomes and equal efficacy for 1-year outcomes (93). Women seem to suffer from more side effects of (sleep alterations, constipation, and weight loss) than men, but this difference did not influence the treatment success rates (94).
In general, data from International Tobacco Control Four Country Survey suggest that smoking cessation medications may narrowing gender related differences in smoking cessation success rates (90). Successfully quitting smoking was lower in women than in men who did not use any medication (13% women vs. 20% men), and there was no difference when medications were used (90).
Taken together, these contradictory results emphasize the need of further investigations about sex/gender differences in medication use and effectiveness in smoking cessation.
Discussion
We acknowledge that no systematic approach was performed in the literature search and no formal qualitative appraisal of the retrieved evidence; nevertheless, we have summarized below some considerations, and suggestions for future research in the field.
Even if recent GLOBOCAN 2020 data show that lung cancer is less frequent in females than in males, today the difference between them is narrowing above all in industrialized countries (8). Tobacco smoking changes over time primarily explain the trends of lung cancer morbidity and mortality in different populations or groups of populations as well as in females and in males (1,22,43). In addition, females seem to be more susceptible to carcinogens in tobacco smoke, and also hormonal factors and the use of HRT are suggested to increase susceptibility to lung cancer and to potentially aggravate lung cancer disease (5,21,22). Thus, growing scientific evidence discussed in this review point out the importance of considering sex/gender differences in the prevention, diagnosis and treatment of lung cancer (44,65,66,70,90). Consequently, sex/gender should be considered as a factor when designing research studies.
Fortunately, these differences are becoming clearer since scientific research and advancements in medicine, toxicology and epidemiology are leading to understand more about lung development, physiology, pathology and risk factors. Even if biologic mechanisms have yet to be fully understood, the publications above mentioned suggest that female sex hormones, not only HRT especially estrogen, may have a role in the development or in the prognosis worsening of lung cancer (5,21,22). Therefore, more research is needed to clearly deepen the role of the hormonal factors in lung physiology as well as pathology.
It is advisable that researchers will discover further sex/gender differences that can be used to address the overall treatment and to personalize lung cancer care.
Genetic changes related to cancer have been more detected in women than in men. Many novel therapies target these genetic alterations such as epidermal growth factor receptor (EGFR) and BRAF mutations, anaplastic lymphoma kinase (ALK), and reactive oxidative species (ROS) rearrangements (95). As regards non-small cell lung cancer, it is particularly advisable to have gene profiling (molecular profiling) done on these tumors, and this is especially important in women who might benefit more from target-oriented stratified medical treatments (21).
Concluding, there is increasing evidence which proves the existence of different risk in development and evolution of lung cancer in women and in men. As above mentioned, hormonal, genetic and metabolic factors probably underlie the highest susceptibility to the effects of tobacco smoke carcinogens in women. It is not clear whether observed modification is a result of sex-linked biological differences, sex differences in metabolic activation or detoxification of environmental carcinogens (96) or gender differences in activity patterns, co-exposures to risk factors, or a combination of these factors (37).
Thus, the significance of sex as a separate contributing factor should be considered in lung cancer prognosis and treatment management.
Conclusions
The incidence of lung cancer among females is still globally increasing and especially in those who do not smoke. Tobacco smoking exposure does not fully explain the natural history of lung cancer: other environmental risk factors and different individual susceptibility play a role, even if their interaction is not completely understood.
Engagement in advocacy for preventing initiation of tobacco use, promoting cessation, eliminating exposure to ETS, implementing clean air policies must be increased taking into account not only sex differences but also gender differences in activity patterns, co-exposures or exposure measurement accuracy, or combination of these factors.
In this perspective, it is highly advisable that future preventative strategies are started as early as possible, mainly implementing school-based programs for lung cancer prevention.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Editta Baldini and Franca Melfi) for the series “Lung Cancer in Women: From Epidemiology to Therapy” published in Precision Cancer Medicine.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-54/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-54/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-54/coif). The series “Lung Cancer in Women: From Epidemiology to Therapy” was commissioned by the editorial office without any funding or sponsorship. LC reports personal fees from Sanofi, Guidotti, GSK, Firma, Chiesi, as well as grants and personal fees from Italian Ministry of Health, Tuscany Region, GSK, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torre LA, Islami F, Siegel RL, et al. Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 2017;26:444-57. [Crossref] [PubMed]
- Thun MJ, Carter BD, Feskanich D, et al. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351-64. [Crossref] [PubMed]
- Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004;328:1519. [Crossref] [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global Epidemiology of Lung Cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Schveigert D, Krasauskas A, Didziapetriene J, et al. Smoking, hormonal factors and molecular markers in female lung cancer. Neoplasma 2016;63:504-9. [Crossref] [PubMed]
- Stapelfeld C, Dammann C, Maser E. Sex-specificity in lung cancer risk. Int J Cancer 2020;146:2376-82. [Crossref] [PubMed]
- Lopes-Ramos CM, Quackenbush J, DeMeo DL. Genome-Wide Sex and Gender Differences in Cancer. Front Oncol 2020;10:597788. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Pito and Doll. King Olav V prize for outstanding cancer research. Science Blog, 2002, Available online: https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm
- World Health Organization. WHO global report on trends in prevalence of tobacco smoking 2000–2025, second edition. 2018. Available online: http://apps.who.int/iris/handle/10665/272694
- Jeon J, Holford TR, Levy DT, et al. Smoking and Lung Cancer Mortality in the United States From 2015 to 2065: A Comparative Modeling Approach. Ann Intern Med 2018;169:684-93. [Crossref] [PubMed]
- Carioli G, Bertuccio P, Boffetta P, et al. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann Oncol 2020;31:650-8. [Crossref] [PubMed]
- López-Campos JL, Ruiz-Ramos M, Fernandez E, et al. Recent lung cancer mortality trends in Europe: effect of national smoke-free legislation strengthening. Eur J Cancer Prev 2018;27:296-302. [Crossref] [PubMed]
- Fang JY, Dong HL, Wu KS, et al. Characteristics and Prediction of Lung Cancer Mortality in China from 1991 to 2013. Asian Pac J Cancer Prev 2015;16:5829-34. [Crossref] [PubMed]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88:183-92. [Crossref] [PubMed]
- Silveyra P, Fuentes N, Rodriguez Bauza DE. Sex and Gender Differences in Lung Disease. Adv Exp Med Biol 2021;1304:227-58. [Crossref] [PubMed]
- Munnia A, Giese RW, Polvani S, et al. Bulky DNA Adducts, Tobacco Smoking, Genetic Susceptibility, and Lung Cancer Risk. Adv Clin Chem 2017;81:231-77. [Crossref] [PubMed]
- Mollerup S, Berge G, Baera R, et al. Sex differences in risk of lung cancer: Expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer 2006;119:741-4. [Crossref] [PubMed]
- Hsu LH, Chu NM, Kao SH. Estrogen, Estrogen Receptor and Lung Cancer. Int J Mol Sci 2017;18:1713. [Crossref] [PubMed]
- Donington JS, Colson YL. Sex and gender differences in non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2011;23:137-45. [Crossref] [PubMed]
- Jin K, Wu M, Zhou JY, et al. Tobacco Smoking Modifies the Association between Hormonal Factors and Lung Cancer Occurrence among Post-Menopausal Chinese Women. Transl Oncol 2019;12:819-27. [Crossref] [PubMed]
- Kadota K, Eguchi T, Villena-Vargas J, et al. Nuclear estrogen receptor-α expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 2015;6:27505-18. [Crossref] [PubMed]
- Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 2009;374:1243-51. [Crossref] [PubMed]
- Dresler CM, Fratelli C, Babb J, et al. Gender differences in genetic susceptibility for lung cancer. Lung Cancer 2000;30:153-60. [Crossref] [PubMed]
- Aoun J, Saleh N, Waked M, et al. Lung cancer correlates in Lebanese adults: a pilot case--control study. J Epidemiol Glob Health 2013;3:235-44. [Crossref] [PubMed]
- Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet 2018;391:462-512. [Crossref] [PubMed]
- Le CH, Ko YC, Cheng LS, et al. The heterogeneity in risk factors of lung cancer and the difference of histologic distribution between genders in Taiwan. Cancer Causes Control 2001;12:289-300. [Crossref] [PubMed]
- Sheng L, Tu JW, Tian JH, et al. A meta-analysis of the relationship between environmental tobacco smoke and lung cancer risk of nonsmoker in China. Medicine (Baltimore) 2018;97:e11389. [Crossref] [PubMed]
- Vogeltanz-Holm N, Schwartz GG. Radon and lung cancer: What does the public really know? J Environ Radioact 2018;192:26-31. [Crossref] [PubMed]
- Akhtar N, Bansal JG. Risk factors of Lung Cancer in nonsmoker. Curr Probl Cancer 2017;41:328-39. [Crossref] [PubMed]
- Sun Y, Li Z, Li J, et al. A Healthy Dietary Pattern Reduces Lung Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2016;8:134. [Crossref] [PubMed]
- Simonato L, Agudo A, Ahrens W, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer 2001;91:876-87. [Crossref] [PubMed]
- Tolwin Y, Gillis R, Peled N. Gender and lung cancer-SEER-based analysis. Ann Epidemiol 2020;46:14-19. [Crossref] [PubMed]
- Consonni D, Carugno M, De Matteis S, et al. Outdoor particulate matter (PM10) exposure and lung cancer risk in the EAGLE study. PLoS One 2018;13:e0203539. [Crossref] [PubMed]
- Hamra GB, Laden F, Cohen AJ, et al. Lung Cancer and Exposure to Nitrogen Dioxide and Traffic: A Systematic Review and Meta-Analysis. Environ Health Perspect 2015;123:1107-12. [Crossref] [PubMed]
- Clougherty JE. A growing role for gender analysis in air pollution epidemiology. Environ Health Perspect 2010;118:167-76. [Crossref] [PubMed]
- Kurmi OP, Arya PH, Lam KB, et al. Lung cancer risk and solid fuel smoke exposure: a systematic review and meta-analysis. Eur Respir J 2012;40:1228-37. [Crossref] [PubMed]
- Jia PL, Zhang C, Yu JJ, et al. The risk of lung cancer among cooking adults: a meta-analysis of 23 observational studies. J Cancer Res Clin Oncol 2018;144:229-40. [Crossref] [PubMed]
- Kim CH, Lee YC, Hung RJ, et al. Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the International Lung Cancer Consortium (ILCCO). Int J Cancer 2014;135:1918-30. [Crossref] [PubMed]
- Center for Disease Control and Prevention. Lung Cancer Among People Who Never Smoked. Last update October 18, 2021. Available online: https://www.cdc.gov/cancer/lung/nonsmokers/index.htm
- Konstantinou E, Fotopoulou F, Drosos A, et al. Tobacco-specific nitrosamines: A literature review. Food Chem Toxicol 2018;118:198-203. [Crossref] [PubMed]
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 2015;4:327-38. [PubMed]
- Trama A, Boffi R, Contiero P, et al. Trends in lung cancer and smoking behavior in Italy: an alarm bell for women. Tumori 2017;103:543-50. [Crossref] [PubMed]
- Shimatani K, Ito H, Matsuo K, et al. Cumulative cigarette tar exposure and lung cancer risk among Japanese smokers. Jpn J Clin Oncol 2020;50:1009-17. [Crossref] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum 2004;83:1-1438. [PubMed]
- Lipfert FW, Wyzga RE. Longitudinal relationships between lung cancer mortality rates, smoking, and ambient air quality: a comprehensive review and analysis. Crit Rev Toxicol 2019;49:790-818. [Crossref] [PubMed]
- Kim AS, Ko HJ, Kwon JH, et al. Exposure to Secondhand Smoke and Risk of Cancer in Never Smokers: A Meta-Analysis of Epidemiologic Studies. Int J Environ Res Public Health 2018;15:1981. [Crossref] [PubMed]
- Cardenas VM, Thun MJ, Austin H, et al. Environmental tobacco smoke and lung cancer mortality in the American Cancer Society's Cancer Prevention Study. II. Cancer Causes Control 1997;8:57-64. [Crossref] [PubMed]
- Pirie K, Peto R, Green J, et al. Lung cancer in never smokers in the UK Million Women Study. Int J Cancer 2016;139:347-54. [Crossref] [PubMed]
- Prüss-Üstün A, Wolf J, Corvalán C, et al. Preventing disease through healthy environments: a global assessment of the burden of disease from environmental risks. World Health Organization, 2016.
- Benbrahim-Tallaa L, Baan RA, Grosse Y, et al. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol 2012;13:663-4. [Crossref] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Outdoor Air Pollution. IARC Monogr Eval Carcinog Risks Hum 2016;109:9-444. [PubMed]
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med 2020;41:1-24. [Crossref] [PubMed]
- Turner MC, Andersen ZJ, Baccarelli A, et al. Outdoor air pollution and cancer: An overview of the current evidence and public health recommendations. CA Cancer J Clin 2020; Epub ahead of print. [Crossref] [PubMed]
- Raaschou-Nielsen O, Thorsteinson E, Antonsen S, et al. Long-term exposure to air pollution and mortality in the Danish population a nationwide study. EClinicalMedicine 2020;28:100605. [Crossref] [PubMed]
- Atkinson RW, Butland BK, Anderson HR, et al. Long-term Concentrations of Nitrogen Dioxide and Mortality: A Meta-analysis of Cohort Studies. Epidemiology 2018;29:460-72. [Crossref] [PubMed]
- Gowda SN, DeRoos AJ, Hunt RP, et al. Ambient air pollution and lung cancer risk among never-smokers in the Women’s Health Initiative. Environ Epidemiol 2019;3:e076. [Crossref] [PubMed]
- Hamra GB, Guha N, Cohen A, et al. Outdoor particulate matter exposure and lung cancer: a systematic review and meta-analysis. Environ Health Perspect 2014;122:906-11. [Crossref] [PubMed]
- Turner MC, Cohen A, Jerrett M, et al. Interactions between cigarette smoking and fine particulate matter in the Risk of Lung Cancer Mortality in Cancer Prevention Study II. Am J Epidemiol 2014;180:1145-9. [Crossref] [PubMed]
- Parodi S, Baldi R, Benco C, et al. Lung cancer mortality in a district of La Spezia (Italy) exposed to air pollution from industrial plants. Tumori 2004;90:181-5. [Crossref] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Household use of solid fuels and high-temperature frying. IARC Monogr Eval Carcinog Risks Hum 2010;95:1-430. [PubMed]
- International Agency for Research on Cancer. Indoor emissions from household combustion of coal. In: Personal Habits and Indoor Combustions. 2012.
- Kliucininkas L, Krugly E, Stasiulaitiene I, et al. Indoor–outdoor levels of size segregated particulate matter and mono/polycyclic aromatic hydrocarbons among urban areas using solid fuels for heating. Atmos Environ 2014;97:83-93. [Crossref]
- Jin ZY, Wu M, Han RQ, et al. Household ventilation may reduce effects of indoor air pollutants for prevention of lung cancer: a case-control study in a Chinese population. PLoS One 2014;9:e102685. [Crossref] [PubMed]
- Public Health Service. Office of the Surgeon General. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014.
- Pirie K, Peto R, Reeves GK, et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013;381:133-41. [Crossref] [PubMed]
- Carrozzi L, Falcone F, Carreras G, et al. Life gain in Italian smokers who quit. Int J Environ Res Public Health 2014;11:2395-406. [Crossref] [PubMed]
- Shields PG, Herbst RS, Arenberg D, et al. Smoking Cessation, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1430-68. [Crossref] [PubMed]
- Goldstein AO, Shoenbill KA, Jolly TA. Intensive Smoking Cessation Counseling for Patients With Cancer. JAMA 2020;324:1401-3. [Crossref] [PubMed]
- Pistelli F, Aquilini F, Falaschi F, et al. Smoking Cessation in the ITALUNG Lung Cancer Screening: What Does "Teachable Moment" Mean? Nicotine Tob Res 2020;22:1484-91. [Crossref] [PubMed]
- Walker NJ, van Woerden HC, Kiparoglou V, et al. Gender difference and effect of pharmacotherapy: findings from a smoking cessation service. BMC Public Health 2016;16:1038. [Crossref] [PubMed]
- West R, Evins AE, Benowitz NL, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction 2018;113:1507-16. [Crossref] [PubMed]
- Smith PH, Bessette AJ, Weinberger AH, et al. Sex/gender differences in smoking cessation: A review. Prev Med 2016;92:135-40. [Crossref] [PubMed]
- Jarvis MJ, Cohen JE, Delnevo CD, et al. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control 2013;22:356-60. [Crossref] [PubMed]
- Jarvis MJ. The association between having children, family size and smoking cessation in adults. Addiction 1996;91:427-34. [Crossref] [PubMed]
- US Department of Health and Human Services. Women and smoking: a report of the Surgeon General. Atlanta, Georgia: US Department of Health and Human Services, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2001.
- Xu J, Azizian A, Monterosso J, et al. Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nicotine Tob Res 2008;10:1653-61. [Crossref] [PubMed]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, et al. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res 2006;8:627-38. [Crossref] [PubMed]
- McKee SA, O'Malley SS, Salovey P, et al. Perceived risks and benefits of smoking cessation: gender-specific predictors of motivation and treatment outcome. Addict Behav 2005;30:423-35. [Crossref] [PubMed]
- Pirie PL, Murray DM, Luepker RV. Gender differences in cigarette smoking and quitting in a cohort of young adults. Am J Public Health 1991;81:324-7. [Crossref] [PubMed]
- Etter JF, Prokhorov AV, Perneger TV. Gender differences in the psychological determinants of cigarette smoking. Addiction 2002;97:733-43. [Crossref] [PubMed]
- Chenoweth MJ, Novalen M, Hawk LW Jr, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev 2014;23:1773-82. [Crossref] [PubMed]
- Murray RP, Johnston JJ, Dolce JJ, et al. Social support for smoking cessation and abstinence: the Lung Health Study. Lung Health Study Research Group. Addict Behav 1995;20:159-70. [Crossref] [PubMed]
- Ziedalski TM, Ruoss SJ. Smoking cessation: techniques and potential benefits. Thorac Surg Clin 2005;15:189-94. [Crossref] [PubMed]
- Lancaster T, Stead L, Silagy C, et al. Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library. BMJ 2000;321:355-8. [Crossref] [PubMed]
- Cahill K, Lindson-Hawley N, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016;CD006103. [Crossref] [PubMed]
- Munafò M, Bradburn M, Bowes L, et al. Are there sex differences in transdermal nicotine replacement therapy patch efficacy? A meta-analysis. Nicotine Tob Res 2004;6:769-76. [Crossref] [PubMed]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine Tob Res 2008;10:1245-50. [Crossref] [PubMed]
- Smith PH, Kasza KA, Hyland A, et al. Gender differences in medication use and cigarette smoking cessation: results from the International Tobacco Control Four Country Survey. Nicotine Tob Res 2015;17:463-72. [Crossref] [PubMed]
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction 2004;99:1462-9. [Crossref] [PubMed]
- Dale LC, Glover ED, Sachs DP, et al. Bupropion for smoking cessation: predictors of successful outcome. Chest 2001;119:1357-64. [Crossref] [PubMed]
- McKee SA, Smith PH, Kaufman M, et al. Sex Differences in Varenicline Efficacy for Smoking Cessation: A Meta-Analysis. Nicotine Tob Res 2016;18:1002-11. [Crossref] [PubMed]
- Castellani V, Gonçalves PD, Castaldelli-Maia JM, et al. Investigating gender differences for effectiveness and side effects of varenicline during smoking cessation treatment. Rev Assoc Med Bras (1992) 2020;66:146-52. [PubMed]
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45-52. [Crossref] [PubMed]
- Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 2011;32:605-44. [Crossref] [PubMed]
Cite this article as: Baldacci S, Maio S, Meschi C, Chimera D, Tagliaferro S, Angino AA, Silvi P, Pistelli F, Carrozzi L. A narrative review of epidemiology and prevention of lung cancer: sex/gender differences? Precis Cancer Med 2022;5:23.

