
KRAS G12V 突变作为达拉非尼和曲美替尼治疗BRAF V600E突变的非小细胞肺癌获得性耐药的机制
简介
近年来,针对非小细胞肺癌(NSCLC)的分子靶向治疗取得了显著进展。NSCLC的致癌因素之一是突变的BRAF,这是一种丝氨酸-苏氨酸激酶,属于RAF激酶家族,与MEK-ERK信号级联直接相互作用。据报道,3%~4%的NSCLC患者[1,2]存在BRAF突变,其中BRAF V600E突变约占所有BRAF突变的一半[3]。
据报道,BRAF抑制剂达拉非尼和MEK抑制剂曲美替尼联合治疗转移性BRAF V600E突变的NSCLC患者的总缓解率(ORR)为64%,无进展生存期(PFS)为10.9个月,并且在一线方案中ORR为63%,在后线方案中PFS为9.7个月[4,5],因此,达拉非尼和曲美替尼联合治疗是目前此类患者的标准治疗方案。然而,与转移性EGFR突变NSCLC的情况类似,缓解持续时间有限且可预见存在获得性耐药。
在一些BRAF V600E突变的NSCLC患者中报告了对达拉非尼单药治疗或达拉非尼和曲美替尼联合治疗的耐药机制,这些机制大致涉及MEK1 K57、KRAS G12D、KRAS G12V、KRAS Q61R、NRAS Q61K和NRAS Q61R突变[6-9]。然而,非小细胞肺癌患者对达拉非尼和曲美替尼联合治疗的耐药机制尚未得到充分描述。
在这里,我们描述了涉及KRAS G12V突变地接受达拉非尼和曲美替尼联合治疗的BRAF V600E突变患者的获得性耐药机制。我们根据CARE报告清单(可在 https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/rc 获得)提出以下案例。
案例展示
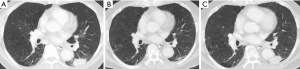
患者是一名70岁的亚洲男性,吸烟史,被诊断为Ⅳ期NSCLC,腺癌亚型。自2018年以来左肺病灶转移至多个纵隔淋巴结以及对侧肺一个单独结节。超声引导下经支气管穿刺活检左肺门淋巴结发现腺癌。表皮生长因子突变、间变性淋巴瘤激酶(ALK)和ROS1重排的初步分子检测为阴性。随后,基于组织的二代测序(NGS)和Oncomine Dx Target Test CDx显示BRAF V600E突变,临床试验设置的Foundation One Liquid也显示BRAF V 600E和TP53 I255S突变。他接受了达拉非尼和曲美替尼联合治疗并取得了疗效,左下叶原发灶、纵隔淋巴结和多发肺转移灶均缩小。2021年1月,他的肺肿瘤原发灶出现进展,并在右叶出现多个新的肺结节(图1)。血浆测序(Guardant360®CDx)显示,除了原始BRAF V600E和TP53 I255S突变(变异等位基因频率均为0.2%)外,还出现了KRAS G12V突变(变异等位基因频率的0.3%),未发现对达拉非尼和曲美替尼耐药的其他基因组机制。疾病进展后,患者已接受卡铂和培美曲塞治疗,卡铂和培美曲塞部分起效,原发肿瘤缩小。
本研究中进行的所有程序均符合机构和/或国家研究委员会的伦理标准以及赫尔辛基宣言(2013年修订)。从患者处获得书面知情同意书以出版本病例报告和随附图像。书面同意的副本可供本刊编辑部审阅。
讨论
携带BRAF V600E突变的NSCLC患者数量相对较小,只有少数病例报道了获得性耐药机制,对复发性分子畸变的识别将有助于为该患者群体开发新的治疗策略。
与使用靶向药物治疗的其他晚期NSCLC类似,耐药性几乎总是会出现。在BRAF突变黑色素瘤中报告了对联合BRAF和MEK抑制剂的多种耐药机制。这些包括RAS/RAF/MAPK通路的二次突变,例如BRAF扩增、BRAF剪接变体和KRAS和NRAS中的功能增益突变[10]。KRAS位于BRAF的上游,其激活可能导致PI3K/AKT/mTOR通路的激活,从而导致肿瘤进展。
在目前报道的患者中,继发性KRAS G12V突变被确定为对达拉非尼和曲美替尼联合治疗的耐药机制。已报道7例对达拉非尼单药治疗或达拉非尼联合曲美替尼治疗产生获得性耐药的病例[6-9],其中1例涉及KRAS G12V突变。在文献综述中,细胞周期蛋白依赖性激酶(CDK)4和CDKN2缺失或无义突变的表达水平升高是非RAS/RAF/MAPK畸变的耐药机制[9,11]。
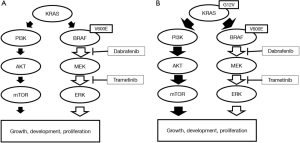
本病例表明KRAS G12V突变存在于一个微小的亚克隆中,联合治疗导致KRAS G12V阳性亚克隆的选择性生长,通过激活PI3K/AKT/mTOR通路导致肿瘤进展(图2)。我们案例的限制是无法基于肿瘤组织的耐药性进行分子分析。基于血浆的NGS检测不能排除不确定潜能的克隆造血。然而,我们的患者在治疗前的血浆NGS检测中没有显示KRAS突变,但在耐药后的血浆NG检测中检测到KRAS变异。因此,我们认为这种耐药后的KRAS突变与克隆造血无关。
综上所述,我们在一例携带BRAF V600E突变的患者中确定了达拉非尼和曲美替尼联合治疗的耐药机制涉及KRAS G12V突变。克服这种耐药性的最优方法仍不清楚,需要进一步努力制定和开发新的治疗策略和药物组合来克服耐药性。
Acknowledgments
We thank LC-SCRUM-Asia and its operation staff for the gene analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-55/coif). KY serves as an unpaid editorial board member of Precision Cancer Medicine from September 2019 to August 2023. KY reports research support from AstraZeneca, Eli Lilly, Pfizer, Daiichi Sankyo, Abbvie, Taiho, Takeda and MSD, and personal fees (honoraria) from AstraZeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Janssen, Eli Lilly, Taiho, Novartis, Kyowa Kirin and Boehringer Ingelheim. SM reports research support from Chugai, Novartis, Eli Lilly, Merck and MSD, and personal fees (honoraria) from AstraZeneca, Chugai, Novartis, Pfizer and Eli Lilly. HI reports research support from Amgen and personal fees (honoraria) from Ono. YS reports research support from MSD, and personal fees (honoraria) from Ono, Pfizer, Chugai, Novartis, Eli Lilly, Bristol-Myers Squibb, AstraZeneca and Taiho. TS reports personal fees (honoraria) from Chugai and AstraZeneca. KN reports research support from Chugai and Takeda, and personal fees (honoraria) from Pfizer, Chugai, Takeda, Novartis and AstraZeneca. YZ reports research support from AstraZeneca, MSD, Daiichi-Sankyo and personal fees (honoraria) from Pfizer, Chugai, Takeda, AstraZeneca, Bristol-Myers Squibb, Ono pharmaceutical, Boheringer-Ingelheim, Taiho, Novartis, MSD and Eli Lilly. KG reports research support from Boehringer Ingelheim, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Guardant Health, Janssen, Kyowa Kirin, Life Technologies Japan, MSD, Novartis, Ono, Otsuka, Pfizer, Taiho and Takeda, and personal fees (honoraria) from Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, Haihe Biopharma, Ignyta, Janssen, KISSEI, Kyowa Kirin, LOXO Oncology, Medical & Biological Laboratories, Merck Biopharma, Merus, MSD, Ono, Pfizer, Sumitomo Dainippon Pharma, Shanghai Haihe, Sysmex Corporation, Taiho, Takeda, and Xcoo. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046-51. [Crossref] [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Planchard D, Besse B, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol 2016;17:984-93. [Crossref] [PubMed]
- Rudin CM, Hong K, Streit M. Molecular characterization of acquired resistance to the BRAF inhibitor dabrafenib in a patient with BRAF-mutant non-small-cell lung cancer. J Thorac Oncol 2013;8:e41-2. [Crossref] [PubMed]
- Niemantsverdriet M, Schuuring E, Elst AT, et al. KRAS Mutation as a Resistance Mechanism to BRAF/MEK Inhibition in NSCLC. J Thorac Oncol 2018;13:e249-51. [Crossref] [PubMed]
- Abravanel DL, Nishino M, Sholl LM, et al. An Acquired NRAS Q61K Mutation in BRAF V600E-Mutant Lung Adenocarcinoma Resistant to Dabrafenib Plus Trametinib. J Thorac Oncol 2018;13:e131-3. [Crossref] [PubMed]
- Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAFV600E non-small cell lung cancer. Eur J Cancer 2020;132:211-23. [Crossref] [PubMed]
- Cohen JV, Sullivan RJ. Developments in the Space of New MAPK Pathway Inhibitors for BRAF-Mutant Melanoma. Clin Cancer Res 2019;25:5735-42. [Crossref] [PubMed]
- Hirai N, Hatanaka Y, Hatanaka KC, et al. Cyclin-dependent kinase 4 upregulation mediates acquired resistance of dabrafenib plus trametinib in BRAF V600E-mutated lung cancer. Transl Lung Cancer Res 2021;10:3737-44. [Crossref] [PubMed]
杨雨菲
复旦大学附属肿瘤医院(更新时间:2023-05-05)
朱恒
复旦大学附属肿瘤医院(更新时间:2023-05-05)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Oi H, Yoh K, Matsumoto S, Izumi H, Shibata Y, Sakai T, Nosaki K, Zenke Y, Goto K. KRAS G12V mutation as an acquired resistance mechanism after treatment with dabrafenib and trametinib in non-small cell lung cancer harbouring the BRAF V600E mutation: a case report. Precis Cancer Med 2022;5:18.

