
非小细胞肺癌中新型驱动基因的研究进展
介绍
肺癌是全球癌症死亡的主要原因。从组织学上讲,肺癌分为两种主要类型:小细胞肺癌(SCLC)和非小细胞肺癌(NSCLC)。大约80%~85%的病例被诊断为NSCLC,约70%的患者在诊断时患有局部晚期或转移性疾病[1]。肺癌总体5年生存率仅为14%~17%[2],主要由于肺癌在早期阶段的难以检测以及晚期时的难以治疗。然而,近年来免疫检查点抑制剂(ICI)和靶向疗法的引入已经彻底改变了许多NSCLC的自然病程,使得患者的生存率、耐受性和生活质量都得到了极大的改善。而其中表皮生长因子受体(EGFR),间变性淋巴瘤激酶(ALK)和c−ros肉瘤致癌基因1(ROS1)这些驱动基因突变的发现,标志着NSCLC治疗的范式转变。现今,广泛的分子谱分析技术揭示了NSCLC的分子异质性,推动NSCLC治疗药物研发进入新阶段。新型B−Raf原癌基因(BRAF)的抑制剂、RET抑制剂、间充质至上皮过渡因子(MET)抑制剂及神经营养性酪氨酸激酶(NTRK)1−3的抑制剂在NSCLC治疗上已经显示出其有效性,并在几年内获得了美国食品药品监督管理局(FDA)和欧洲药品管理局(EMA)的加速批准。许多其他已鉴定的突变现在是潜在治疗靶点的候选者,目前正在评估这些新靶点的选择性抑制剂的疗效。因此,我们系统性综述了NSCLC中新可能靶点的临床试验,以及PubMed、Web of Science和Scopus数据库2016年1月至2021年6月发表的相关文献,以分析当前最新NSCLC药物研发现状。在我们的检索策略中主要使用了以下搜索词:非小细胞肺癌或肺癌、篮子方案或伞式方案或生物标志物驱动或精准医学或精准肿瘤学或分子谱分析或基因组谱分析非回顾性。此外,本文还简要综述了美国临床肿瘤学会(ASCO)、欧洲肿瘤内科学会(ESMO)大会和世界肺癌会议(WCLC)等重大会议上报告的相关研究摘要。我们使用叙述性评论报告清单来介绍以下文章(可在https://pcm.amegroups.com/article/view/10.21037/pcm-21-19/rc获取)。
Kirsten大鼠肉瘤病毒癌基因同源物(KRAS)
组织学和分子学特征
KRAS突变是NSCLC中最普遍的突变之一[3]。它在腺癌中更为广泛,在高加索患者中的患病率为20%~40%,在亚洲患者中为2%~10%,这与两个人群中EGFR突变的相反频率趋势形成鲜明对比[4]。KRAS突变性肺癌以明确的临床病理学特征为标志,它们通常与浸润性粘液腺癌(invasive mucinous adenocarcinoma,IMA)相关,相比于混合黏液及非黏液癌,它更常见于纯黏液癌,它们也发生在具有实体瘤样的肺腺癌(LUAC)中,且在KRAS突变的LUAC中也经常检测到其他基因的体细胞突变。根据RNA测序结果显示的共性突变基因以及LUCA的生物学和免疫特性,LUAC可分为三个亚型[5]:第一类亚型为丝氨酸/苏氨酸激酶11(STK11)/肝激酶B1(LKB1)基因发生失活突变的STK11/LKB1亚型(KL亚型),该亚型显示LKB1−AMP活化蛋白激酶(AMPK)轴的功能失活以及对氧化、蛋白毒性和高能应激的适应性。此外,该亚型还富集Kelch样ECH关联蛋白1(kelch-like ECH-associated protein 1,Keap1),而却显示低T细胞浸润和程序性死亡-1配体(PD-L1)的低表达,这表明KL亚型相对缺乏免疫系统参与。
相反,TP53共突变的KP亚型的特征是CD8+ T细胞浸润密度高和PD-L1的高表达,并伴随着JAK/STAT炎症通路的激活和免疫耐受/逃逸基因的富集。以上这两亚型在吸烟者中更常见,其中KRAS突变肿瘤在基因组上更复杂,并且比从不吸烟者的肿瘤具有更高的突变负担[6]。第三组KC亚型携带细胞周期蛋白依赖性激酶抑制基因2A/B(CDKN2A/B)的双等位基因丢失,其免疫组织化学染色上甲状腺转录因子−1(TTF−1)表达阴性,而较多呈现黏液组织学形态和胃肠道分化相关基因的激活[5]。同样的,Jurmeister等人已经证明,具有肠道形态学和免疫组织化学特征的LUAC具有明显的分子特征[7]。他们分析了IMA、肺肠腺癌(PEAD)和肺胶体腺癌(CAD)的分子特征,发现KRAS突变是IMA中最常见的遗传改变,其次是CD74−NRG−1易位。而PEAD似乎更常发生在重度吸烟者中,显示出最高的肿瘤突变负担。TP53和腺瘤性息肉病大肠杆菌(APC)突变在PEAD中比在IMA或CAD中更常见,而MYC扩增经常发生在CAD中。大多数突变发生在密码子12和13中。最常见的突变是KRASG12C(8%~13%),其次是KRASG12V(7%)和KRASG12D[8]。KRASG12C突变常见于吸烟者,相比之下,KRASG12D在非吸烟者中更常见。
药物开发和临床试验
近年来,许多研究试图靶向KRAS突变或其下游途径,但研究结果并不理想。许多研究者评估了MEK、MET、表调节蛋白、WT1、GATA2或NF-kb等KRAS途径的基因作为靶标的可行性,但它们都没有达到令人满意的结果[9,10]。而对于KRAS突变的NSCLC,目前的研究重点集中在KRASG12C突变[11]。表1中展示了与KRASG12C抑制剂相关的试验研究的完整概况。其中,Sotorasib和MRTX849在反应率(RR)和疾病控制率(DCR)方面在疾病早期显示出可喜结果[12,17]。KRASG12C可以在GDP和GTP形式之间积极循环,保持与其下游效应子的相互作用。突变的半胱氨酸位于开关II区域的口袋(P2)旁边。P2口袋只存在于KRAS的非活性的GDP结合构象中。Sotorasib(AMG510)是一种不可逆的靶向KRASG12C的小分子抑制剂,将KRAS锁定在其非活性的GDP结合构象中。通过靶向突变的半胱氨酸残基,这种机制允许通过阻断蛋白质的无活性构象来特异性抑制蛋白表达。2019年国际肺癌研究协会(IASLC)首次在巴塞罗那举行的世界肺癌大会(WCLC)上公开汇报了关于KRAS突变实体瘤患者的Ⅰ期临床试验,该试验结果于2020年正式发表[12]。该试验的肺癌组中,32%的患者显示治疗效果,88%的患者的肺癌得到控制,其无进展生存期(PFS)为6.3个月。该药物Ⅱ期临床试验的数据近期已提交至2021年IASLC世界会议上,共纳入了126例KRASG12C突变的NSCLC患者[13],且纳入的患者必须已经接受了至少两轮对转移性疾病的治疗药物。患者的总体反应率(ORR)为37%,DCR为81%。中位反应持续时间为10.0个月,中位PFS为6.8个月。该药物Ⅲ期的临床试验“Codebreak 200”目前正在招募中,与二线治疗药物中的docetaxel进行比较[14]。MRTX849(adagrasib)是另一个KRASG12C不可逆抑制剂,也在近期的Ⅰ/Ⅱ期试验中显示出较好的结果,实现了43%的ORR和96%的DCR[15]。Ⅱ期KRYSTAL−7试验正在进行,以评估adagrasib与Pembrolizumab联合治疗KRASG12C突变的NSCLC患者的疗效,而Ⅲ期KRYSTAL−12研究正在比较adagrasib与docetaxel在还未经治疗患者中的疗效[16,18]。这些研究都显示出了靶向KRAS突变癌症的复杂性,这可能源于许多不同的畸变和共突变都有可能调节肿瘤生物学性状及对治疗的反应。近期一项对38例adagrasib耐药性患者的研究数据提示了一些主要的肿瘤逃逸机制[19]。这38例患者中,27例患有肺癌,10例患有结直肠癌,1例患有阑尾癌。较为明确的肿瘤逃逸机制主要包括MET扩增、NRAS、BRAF、有丝分裂原活化的蛋白激酶激酶1(MAP2K1)、RET的突变、ALK、RET、BRAF、RAF1和成纤维细胞生长因子受体(FGFR)−3的融合,以及神经纤维瘤病1型(NF1)和磷酸酶和张力素同源物(PTEN)的功能丧失突变。此外,两名LUAC患者表现出组织学转化为鳞状细胞肺癌(SqCC),但没有确定其他耐药机制。

Full table
PIK3CA
组织学和分子学特征
磷脂酰肌醇3激酶(PI3K)家族是复杂的细胞内信号通路的一部分,涉及蛋白激酶B(PKB),也称为AKT,以及雷帕霉素(mTOR)的靶标,称为PI3K/AKT/mTOR信号通路。它在细胞内信号传导中起着至关重要的作用,并且参与许多细胞过程,如生长、代谢和细胞周期进展。因此,影响该信号通路的体细胞突变可能导致增殖失调和癌症[20]。PI3K包括调节(p85)和催化(p110)亚基,编码催化部分三个基因分别为PIK3CA、PIK3CB和PIK3CD,第一个基因为各种类型的癌症中最常见的突变基因[21]。PIK3CA突变占NSCLC的2%~7%,更常见于鳞状细胞癌和亚洲人群[22,23],因此其与吸烟史具有高度相关性。值得注意的是,在一项针对3908例患者的系统评价和荟萃分析中,只有淋巴结转移状态与PIK3CA突变呈正相关[24]。这种突变对生存率的影响尚不清楚,尽管许多作者认为这种亚型患者的预后较差[24,25]。有趣的是,在临床前模型中,即使PI3K/AKT/mTOR信号通路的负调节因子PTEN发生突变,PIK3CA突变本身也不具有启动和促进肿瘤发生的能力。体外和体内实验均表明,PIK3CA突变需与其他致癌驱动因素(如BRAFV600E、KRASG12D和TP53沉默),共同调控肿瘤的稳态与进展。只有联合治疗才能改善治疗效果[26,27]。这一观察结果反映在临床实践中,在从NSCLC患者收集的组织标本中,额外的致癌驱动因素畸变率从57%到75%以上不等[28]。
药物开发和临床试验
迄今为止,早期临床试验的现有数据尚未提供令人满意的结果,它们主要研究了单个PI3K/AKT/mTOR信号通路抑制剂或两个抑制剂联合应用的影响,且通常与标准护理治疗相结合[29]。Pictilisib是一种泛I类PI3K抑制剂,一项IA/IB期试验对其进行了单独或与化疗联合使用的疗效评估,但其疗效结果尚未在Ⅱ期研究中得到证实[30]。而像PX−866和buparlisib(BKM120)等其他泛类抑制剂的Ⅰ/Ⅱ期临床试验的数据同样未能证明药物对患者生存率的改善[31,33]。而一项Ⅱ期LUNG−MAP临床试验研究了选择性PI3K p110α、p110γ和p110δ亚型抑制剂[即Taselisib(GDC-0032)]对21例具有PIK3CA突变患者的疗效,但这些患者未能达到其主要终点,因此该试验在中期分析后因无效而关闭[34]。一项Ⅰ期篮式研究显示,Taselisib在患有PIK3CA突变的各类不同癌种人群中的疗效有限[35]。此外,AKT抑制剂的疗效在一项Ⅰ/Ⅱ期试验中被研究,对15名患者应用Perifosine治疗,有一病例尚未明确为部分应答(PR),两例患者达到疾病稳定(SDs),但随后的Ⅱ期试验的结果尚未公布[36]。研究者们还研究了另一种化合物MK−2206与erlotinib联合治疗对先前接受过EGFR抑制剂治疗患者的疗效。其中,EGFR野生型患者的中位PFS为4.6个月,EGFR突变患者的中位PFS为4.4个月[37]。还有许多研究在NSCLC患者中测试了mTOR抑制剂的疗效,大多数Ⅰ/Ⅱ期试验是基于药物已被证明对其他癌种的疗效而进行的。依维莫司是一种mTOR复合物1(mTORC1)抑制剂,其单独或与化疗、靶向治疗一起进行疗效评估的结果尚可,但未进入Ⅲ期试验[38-41]。西罗莫司与培美曲塞联合治疗复发性转移性NSCLC时显示出潜在的益处[42]。而Temsirolimus与口服ERBB2抑制剂Neratinib联合使用对ERBB2突变肺癌患者的疗效却有限[43]。此外,研究者们还开发了mTORC1和mTORC2复合物的抑制剂,Vistusertib(AZD2014)与紫杉醇联合使用在经过治疗的鳞状NSCLC患者中显示出较显著的RR(33%)[44]。表2提供了与PI3K/AKT/mTOR信号通路研究相关试验的完整视图。值得注意的是,这些药物尚未获得治疗NSCLC的批准,并且缺乏可靠的疗效数据支持。目前,选择性抑制PI3K/AKT/mTOR信号通路未能改善肺癌患者ORR和生存率的原因仍不完全清楚。如前所述,PI3K突变似乎发生在多步骤致癌过程的后期。它可能通过在不同肿瘤区域之间发展为耐药亚克隆来促进肿瘤内异质性发展,而这些亚克隆具有更高的突变负担和不同的生存途径[28]。因此,需要进一步的研究来找到靶向PIK3CA突变肿瘤的治疗最佳策略。
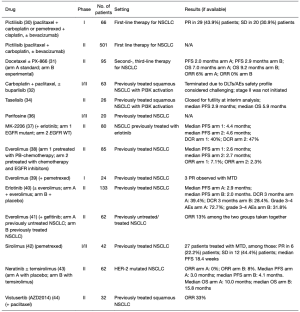
Full table
神经调节素−1(NRG−1)
组织学和分子学特征
NRG−1是生长因子大家族的一部分,其结构中呈现出类似EGF的共识序列,这有利于它们与ERBB跨膜受体酪氨酸激酶(RTKs)的结合[45]。ERBB受体在细胞增殖、发育和分化中保持重要作用,并在上皮、神经元和心血管系统中表达[46]。该家族细分为四个成员:ERBB1(也称为EGFR)、ERBB2(也称为HER2)、ERBB3和ERBB4,它们在结构和激活类型方面略有不同[47]。它们由细胞外配体结合结构域、并列结构域和细胞内激酶结构域组成,其允许通过不同的途径进行信号传导,如RAS/RAF/mitogen活化蛋白激酶(MAPK)或已经提到的PI3K/AKT/mTOR[48-49]。因此,这些受体的异常激活可能导致细胞异常生长和癌症发展[50]。配体结合后,这些受体形成同源和异源二聚体以继续进行激酶信号传导,只有ERBB1和ERBB4是自主的。ERBB2不能结合配体,ERBB3则缺乏激酶活性[51-52]。这些蛋白质的功能激活依赖于与其他家族成员的异源二聚化。奇怪的是,ERBB2−ERBB3被认为是最具转化性和有丝分裂性的一对[53-54]。NRG−1由同源基因编码,产生六种蛋白质(Ⅰ~Ⅵ)和至少31种亚型[55]。其中,Ⅲ型NRG−1呈现膜锚定的表皮生长因子(EGF)样结构域,其可能以自分泌和旁分泌方式起作用[55]。最近的研究表明,NRG−1过表达以及ERBB3异常信号传导可能是临床前模型中癌症发展和维持的原因[56-58]。此外,NRG−1与ERBB3的结合可以诱导ERBB2−ERBB3异源二聚化[59]。LUAC与NRG−1基因的重排有关,特别是黏液亚型LUAC,据报道,在非吸烟女性患者中患病率为0.14%~1.7%。LUAC中发现了多种伴侣基因,如CD74、SDC4、SLC3A2和VAMP2,其中CD74是最常见的一种。文献中报道的唯一一种肺鳞状细胞癌就携带SMAD4−NRG−1融合基因[60]。NRG−1融合导致NRG−1的EGF样结构域的异常表达,NRG−1作为ERBB3(HER3)的配体产生ErbB2/ErbB3异源二聚化和ErbB3磷酸化,从而连续刺激下游PI3K/AKT通路。所有具有CD74−NRG−1融合基因的黏液腺癌都表达磷酸化ErbB3蛋白(pErbB3)。因此,pErbB3的免疫组化已被提出作为对NRG−1融合基因的筛查试验[61]。在LUAC组织模型中,NRG−1基因融合似乎主要发生在8%~32%的IMA中[62]。
药物开发和临床试验
由于NRG−1的分子特征,许多临床前和临床研究对ERBB受体抑制剂进行了疗效评估。在一项临床前模型中,具有CD74−NRG−1融合蛋白的人肺癌细胞暴露于泛ERBB抑制剂阿法替尼和EGFR及HER2抑制剂拉帕替尼后,细胞信号传导和生长都受抑制[63]。在异种移植模型中,抗ERBB3药物GSK2849330和泛ERBB抑制剂Tarloxotinib均显示出抗肿瘤活性[64,65]。其他的一些潜在的NRG−1融合靶向药物也被开发出来,例如Seribantumab(MM−121)、AV−203、9F7−F11和LJM716,但也仅有临床前和Ⅰ期临床试验数据验证其疗效[66,67]。临床实践中使用最广泛研究的化合物是阿法替尼,已有IMA、NSCLC、卵巢癌、胰管腺癌等患者的病例报告显示出其潜在疗效。一项全球多中心的临床研究纳入了80例NRG−1融合阳性的NSCLC患者[68],其中,12例接受Afatinib治疗,ORR为18%,DCR为36%,中位PFS为3.5个月。在Drilon等人[63]的研究中,4例患者在开始治疗后很快便发生了疾病的进展。这提示我们需要了解在这种错综复杂的情况下肿瘤发生耐药的可能机制。而Gay等人[69]在一项纳入404例NSCLC患者的队列研究中发现2名患者患有NRG−1融合阳性癌症(0.5%),一个携带SLC3A2−NRG−1融合,其PFS为12个月,而第二个显示更频繁的CD74−NRG−1,其PFS为10个月。在这些病例中,阿法替尼对受过药物治疗(第一例)和未治疗(第二例)的患者均有效,表明具有潜在和具体的生存获益。值得注意的是,其他ERBB抑制剂也取得了可接受的结果。Lumretuzumab(RG7116)是一种抗ERBB3抗体,已有研究验证了其在2例已受过治疗的SLC3A2−NRG−1融合患者中的疗效,这2例患者都达到了SD,PFS为4个月[70]。Zenocutuzumab(MCLA−128)是一种双特异性抗HER2/ERBB3抗体,目前正在进行针对NRG−1融合癌症患者的Ⅱ期篮式临床试验中进行测试(NCT02912949)。其中一名NSCLC患者正在经历PR,持续4.5个月的PFS[71]。有关NRG−1抑制剂的试验和病例报告的完整视图见表3。

Full table
正是因为我们对非常见基因特征的靶向治疗需求的不断增长,药物重新发现协议试验(DRUP,NCT02925234)旨在将已验证可行的治疗方法应用于具有特定基因突变的患者。类似的,靶向制剂和分析利用注册研究(TAPUR,NCT02693535)预测将招募超过3300例具有13类基因突变的患者。了解ERBB抑制剂获得性耐药的机制是当今所面临的另一个巨大挑战,尽管如此,肿瘤分子图谱的最新进展可以帮助克服这一难点,以满足患者个体化治疗的需求。
ERBB2
组织学和分子学特征
ERBB2突变发生于一小部分NSCLC患者亚型,它们被认定为致癌驱动因素或作为抗性突变出现。具有ERBB2突变的NSCLC患者通常具有低于标准化疗的RR和较短的总生存期(OS)。一般来说,HER2突变可分为三个亚型:突变、扩增和蛋白质过表达。通常来讲,大多数ERBB2过表达的病例是由于基因扩增,但也可能是由于转录机制或蛋白质稳定性增加等转录后调节机制而导致的。ERBB2突变可以在细胞外(外显子5−8)和跨膜(外显子17)结构域中检测到,但它们在酪氨酸激酶结构域(TKD=外显子18−24)中更常见,EGFR也是如此。与EGFR突变类似,TKD中的突变体可以是替换突变、ex19dels突变、框内ex20ins突变或重复突变。其中框内ex20ins突变是最常见的,在2%~10%的LUACs中发现此突变[72-74]。这种突变在非吸烟者和亚洲人群中常见,女性偏多,平均年龄为60岁[75]。ERBB2突变的LUAC常常是中度或低分化的[76]。绝大多数具ERBB2突变的LUAC中发现了ERBB2突变和扩增,然而,只有少数具有HER2突变的样品的免疫组化显示出ERBB2蛋白的过表达,这表明单纯的基因突变似乎与蛋白表达的增加无关。据报道,在所有NSCLC中,2%~5%的腺癌、2%~7%的大细胞癌和1%的鳞状细胞癌的ERBB2拷贝数增加[77]。
如前所述,EGFR和ERBB2属于同一受体家族,具有非常相似的构象和作用机制,它们的外显子20由一个区域组成,即α−C螺旋和其后的环状结构[78],而EGFR和ERBB2的活化状态取决于蛋白的C螺旋是否具有活性构象。当外显子20插入发生时,C螺旋变为永久性的活性构象,从而增强了携带这些突变体的细胞的存活率、侵袭性和致瘤性。大多数插入片段长度为3 bp至12 bp,并且位于外显子的近端区域,在密码子775和881之间。最常见的插入是p.A775_G776insYVMA,其中12 bp片段的插入导致密码子775处氨基酸YVMA的重复[79-80],并且D770−N771insX是EGFR外显子20最常见的突变[81]。这些变化不会增加对EGFR TKI的亲和力,因为它们不涉及ATP结合口袋[82]。相反,它们迫使αC−螺旋进入αC−in位置,引起本构二聚化和活化。
药物开发和临床试验
部分NSCLC患者是预先就存在EGFR突变的,也有部分是在靶向治疗后获得EGFR突变,且经过EGFR TKI治疗后的EGFR突变患者的患病率逐渐增加。最近的研究表明,在对EGFR−TKI治疗产生耐药性的患者中,约有10%~15%的患者发生了ERBB2突变[83]。研究者们已经把不同的TKI和抗ERBB2药物对ERBB2突变性肺癌的治疗效果进行了评估,结果对比差异较大。第二代EGFR抑制剂(阿法替尼、达科米替尼和纳拉替尼)可以不可逆地与EGFR和ERBB2结合[81]。阿法替尼在Niche试验中显示出对ERBB2突变的NSCLC患者的疾病控制程度有限[84]。此外,在最近发表的一项回顾性多中心研究中,阿法替尼对经过ERBB2靶向治疗后具有ERBB2突变的LUAC患者的疗效尚可,中位反应持续时间为6个月[85]。
与其他TKI相比,达科米替尼在EGFR和ERBB2突变NSCLC中呈现了较好的结果。在一项Ⅱ期研究中,26例ERBB2突变或扩增NSCLC患者中的3例患者获得了PR。Neratinib与mTOR抑制剂temsirolimus联合使用在临床前试验中以及随后的Ⅱ期临床研究中都显示出了对具有ERBB2突变的NSCLC患者较好的疗效[87]。一项回顾性研究纳入了欧洲各个中心共101例ERBB2突变的NSCLC患者,其中65例接受了ERBB2靶向药物治疗,包括曲妥珠单抗(n=57)、曲妥珠单抗恩坦辛(T−DM1,n=1)、纳拉替尼(n=14)、阿法替尼(n=11)和拉帕替尼(n=5)。不同的靶向药物治疗后,研究者们观察到不同的临床反应:接受了曲妥珠单抗(曲妥珠单抗和T−DM1)的患者RR为50.9%,PFS为4.8个月(95%CI:3.4~6.5);相反,五名接受拉帕替尼治疗的患者都发生了疾病进展[88]。
莫博替尼(TAK−788)可以选择性地抑制EGFR和ERBB2突变的外显子20,研究者已在体外和体内对其疗效进行了研究[89]。最近,莫博替尼在受过靶向治疗的具有EGFR外显子20插入的NSCLC患者的疗效上取得了不错的临床数据,这也使它获得了FDA的批准。
与其他TKI相比,Poziotinib在体外研究中显示出对具有ERBB2外显子20突变肺癌的疗效最强,这可能由于其体积小和活性强。Poziotinib在体外能有效抑制EGFR或ERBB2外显子20突变的细胞生长,此外,它也在临床试验中展示出其对受过靶向治疗的NSCLC患者不错的临床疗效。一项Ⅱ期临床研究中,Poziotinib治疗后的ORR为27%(95%CI:12%~46%),中位PFS为5.0个月[95%CI:4.0~非可评估(NE)],中位OS为15个月(95%CI:9.0~NE)。但是该药物存在不良反应率较高的问题,总体而言,47%的病例报告了G3皮肤毒性,G3腹泻和20%的G3甲沟炎[91]。
帕齐替尼在ZENITH20−2试验中也取得了可喜的临床结果,其疗效在该研究中得到证实。该试验招募了90例ERBB2外显子20插入患者[92],治疗后的ORR为27.8%(95%CI:18.9%~38.2%),这些试验都达到了临床研究终点,治疗伴随的TKI相关毒性都在可控范围内。
吡咯替尼是一种多靶标TKI,可阻断ERBB1、ERBB2和ERBB4活性。一项Ⅱ期临床试验的数据显示,吡咯替尼对受过靶向治疗的ERBB2外显子20突变的晚期NSCLC患者的临床疗效较好,8例(53.3%)达到PRs和3例达到(20.0%)SD,中位PFS为6.4个月(95%CI:1.60~11.20)[93]。
抗ERBB2药物已成为ERBB2高表达乳腺癌患者的标准治疗方法,现已常规用于携带ERBB2突变的胃癌和结肠癌患者。目前,已有临床前和临床研究评估了抗ERBB2药物对具ERBB2突变的NSCLC的疗效。最近的一项Ⅱ期篮式试验评估了曲妥珠单抗−恩坦辛(T−DM1)在ERBB2突变LUAC中的应用,结果显示ORR为44%[8/18],中位PFS为5个月(95%CI:3~9)。而且,T−DM1对EGFR的外显子20插入、点突变和ERBB2扩增中均具有活性[94]。
曲妥珠单抗deruxtecan(DS−8201a)是一种与拓扑异构酶−抑制剂Exatecan衍生物结合的抗ERBB2抗体,研究者已在体外和体内研究中评估了它对ERBB2突变细胞系的活性作用[95]。最近,一项Ⅰ期临床试验对其进行进一步疗效评估[96],该临床试验招募了具有ERBB2改变的癌症患者,DS−8201a治疗后的中位治疗持续时间为5.5个月(范围为0.69~14.19个月)[97]。Ⅱ期临床研究DESTINY−Lung01的最新结果显示曲妥珠单抗的ORR为55%(95%CI:44%~65%),中位PFS为8.2个月(95%CI:6.0~11.9),中位OS为17.8个月(95%CI:13.8~22.1)[97-98]。
Tarloxotinib是一种共价的泛ERBB酪氨酸激酶抑制剂Tarloxotinib−E的前体药物,其利用肿瘤缺氧在肿瘤微环境中产生高水平的下游效应子,即Tarloxotinib−E[99]。在临床前研究中,Tarloxotinib在携带EGFR ex20ins、HER2 ex20ins、HER2扩增或NRG-1融合的患者衍生细胞系和异种移植模型中表现出较强活性[99]。在RAIN试验中,Tarloxotinib在携带ERBB2激活突变的NSCLC患者中显示出强效的抗肿瘤活性和一定的安全性,获得了22%的ORR,67%的DCR[100]。表4总结了目前研究ERBB2阳性NSCLC抗ERBB2疗法的试验。
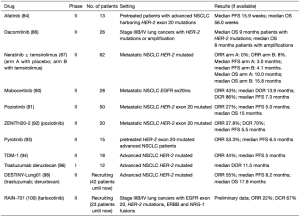
Full table
事实上,使用TKI和抗ERBB2药物获得的临床数据虽然让我们看到其临床应用的希望,但并不完全令人满意,需要努力更好地发现诸如点突变、受体插入、扩增和过表达等不同ERBB2突变类别对不同治疗的反应。
FGFR
组织学和分子学特征
0.2%~6%的NSCLC患者会发生FGFR-1/2/3突变[101-102],表现为扩增、点突变或易位,且在男性和吸烟者中较多发生FGFR的突变,中位诊断年龄为67.5岁(范围为36~89岁)。FGFR−1扩增(8p12)已在9%~22%的鳞状细胞癌(LUSC)中被报道[103-104]。Weiss等人通过高分辨率基因组谱分析了77个LUAC和155个鳞状细胞癌,并确定了FGFR−1仅在高加索人的LUSC中扩增[105]。通过荧光原位杂交(FISH)技术发现,FGFR−1在22%的SqCC样品中扩增。FGFR-1扩增的发生率也与吸烟状况有关,这表明吸烟可能会诱发FGFR-1扩增。关于FGFR-2和FGFR-3突变,Helsten等人描述了3%LUSC中的FGFR-2/3突变以及4%LUAC中FGFR基因异常[106]。在Hibi等人的研究中,FGFR突变的NSCLC患者FGFR-1突变发生率为2.7%,FGFR-2为2.7%,FGFR-3为0及FGFR-4为5.3%[107]。
药物开发和临床试验
在许多癌症中,FGFR都有望成为有效的治疗靶点。FGFR酪氨酸激酶由四种基因(FGFR-1、FGFR-2、FGFR-3、FGFR-4)编码,并参与细胞增殖、迁移、血管生成和上皮间充质转化。FGFR抑制剂已在各种体外研究中显示出疗效,具有FGFR突变的临床前细胞系和患者来源的鳞状NSCLC异种移植模型均表明对FGFR抑制剂治疗敏感。目前,已有一些靶向抑制FGFR的大分子被研发出来:单克隆抗体(如MFGR1877S)、配体陷阱(如FP1039/GSK305223042)、非选择性TKI和选择性TKI[108-110]。几种非选择性TKI抑制剂,如Dovitinib、Nintedanib、Cediranib、Ponatinib、Lucitanib和Pazopanib均已经在FGFR突变的NSCLC患者中进行了临床疗效的评估。然而,所有这些药物都具有较大毒性。这可能是因为它们的非选择性,并且主要依赖于VEGF/VEGFR途径抑制。
与非选择性抑制剂相比,选择性TKIs(如LY2874455、BGJ398、BAY1163877 和JNJ42756493)表现出更好的安全性,仅可能发生的不良反应为高磷血症。在一项IB期研究中,GSK3052230抑制剂与卡铂或多西紫杉醇联合使用,与化疗相关的治疗耐受性良好,并且没有观察到高磷血症、指甲和皮肤毒性等特定不良事件[111]。AZD4547是FGFR-1/2/3的抑制剂,尽管在体外和Ⅰ期试验中获得了良好的结果,但它尚未达到预期的临床疗效。肺图SWOGS1400D的一项Ⅱ期临床子研究中对该分子的临床疗效进行了评估。该研究纳入了铂类治疗失败后FGFR突变的SqCC患者[112],仅有2例患者对治疗有反应。一项正在进行的ERDAFITINIB Ⅱ期试验评估了一个最近被FDA批准用于FGFR突变的尿路上皮癌的药物——Erdafitinib,该临床试验正在招募FGFR 1突变和/或易位的患者[113]。Pemigatinib目前也被一些临床前研究证明在在各种恶性肿瘤中单独或者与化疗或ICI联合使用具有一定疗效[114]。表5总结了目前研究抗FGFR抑制剂治疗FGFR突变NSCLC的临床试验。综上所述,目前仍没有FGFR抑制剂能展现出良好的临床价值,其主要障碍是具有不同FGFR突变的肿瘤对FGFR抑制剂的敏感性不同。临床前研究显示,不同的基因突变患者对不同的FGFR抑制剂治疗后的反应明显不同。关注FGFR突变的多样化和特殊的分子机制是我们找到针对这种基因突变亚组的靶向治疗药物的关键。

Full table
讨论
在个体化医疗时代,围绕单个患者定制治疗方案可能是获得更好结果和更低毒性的正确选择[115-116],肺癌可以被认为是这种创新方法的模型。驱动突变的发现和特定抑制序列的采用使具有特定突变亚型患者实现中位生存成为可能,这在几年前是不可想象的。其中一个典型例子是ALK阳性NSCLC患者的治疗历史,其中位生存期从化疗时代的12个月,到随着新的第二代和第三代ALK抑制剂的出现变为了至少7年以上[117]。可惜的是,仅有一小部分NSCLC患者具有可以应用药物靶向治疗的基因突变[118]。随着研究者们越来越常规地应用全面的基因组分析方法,揭示了肺癌的分子异质性,提示了其他可能的治疗靶点。几年后,随着选择性抑制剂的出现,历史上被认为是不可药理的突变,如KRAS突变,引起了人们的新兴趣。相反,其他突变如PI3CKA和FGFR突变,虽然显示出了它们的临床应用潜力,但尚未取得令人满意的结果。未来几年的目标将是建立一个真实可靠且可复制的精准肿瘤学模型,该模型可以从临床前研究提供可靠的证据,以便在临床试验的后期阶段进行转化。相反,目前研究的风险在于分子数量众多,但支持其疗效的证据不足[119]。
我们的综述旨在重点介绍一些“非常规”NSCLC基因突变的最新研究发现,列出了一些研究结果和正在进行的临床试验。复杂并具异质性的肿瘤中具有的广阔的突变基因,发现这些突变基因,并建立一个以患者为中心的精准肿瘤学新模型,我们就有可能从根本上改变NSCLC的自然病程。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Marco Russano) for the series “Uncommon Mutations in Non-Small Cell Lung Cancer” published in Precision Cancer Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-19/rc
Peer Review File: Available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-19/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://pcm.amegroups.com/article/view/10.21037/pcm-21-19/coif). The series “Uncommon Mutations in Non-Small Cell Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584-94. [Crossref] [PubMed]
- Lu T, Yang X, Huang Y, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res 2019;11:943-53. [Crossref] [PubMed]
- Adderley H, Blackhall FH, Lindsay CR. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019;41:711-6. [Crossref] [PubMed]
- Zhang XC, Wang J, Shao GG, et al. Comprehensive genomic and immunological characterization of Chinese non-small cell lung cancer patients. Nat Commun 2019;10:1772. [Crossref] [PubMed]
- Skoulidis F, Byers LA, Diao L, et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov 2015;5:860-77. [Crossref] [PubMed]
- Redig AJ, Chambers ES, Lydon CA, et al. Genomic complexity in KRAS mutant non-small cell lung cancer (NSCLC) from never/light-smokers v smokers. J Clin Oncol ;34:abstr 9087.
- Jurmeister P, Vollbrecht C, Behnke A, et al. Next generation sequencing of lung adenocarcinoma subtypes with intestinal differentiation reveals distinct molecular signatures associated with histomorphology and therapeutic options. Lung Cancer 2019;138:43-51. [Crossref] [PubMed]
- Ferrer I, Zugazagoitia J, Herbertz S, et al. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer 2018;124:53-64. [Crossref] [PubMed]
- Carter CA, Rajan A, Keen C, et al. Selumetinib with and without erlotinib in KRAS mutant and KRAS wild-type advanced nonsmall-cell lung cancer. Ann Oncol 2016;27:693-9. [Crossref] [PubMed]
- Infante JR, Papadopoulos KP, Bendell JC, et al. A phase 1b study of trametinib, an oral Mitogen-activated protein kinase kinase (MEK) inhibitor, in combination with gemcitabine in advanced solid tumours. Eur J Cancer 2013;49:2077-85. [Crossref] [PubMed]
- Friedlaender A, Drilon A, Weiss GJ, et al. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer Treat Rev 2020;85:101978. [Crossref] [PubMed]
- Hong DS, Fakih MG, Strickler JH, et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med 2020;383:1207-17. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Reck M, Spira A, Besse B, et al. TiP CodeBreak 200: A phase III multicenter study of sotorasib (AMG 510), a KRAS(G12C) inhibitor, versus docetaxel in patients with previously treated advanced non-small cell lung cancer (NSCLC) harboring KRAS p.G12C mutation. Ann Oncol 2020;31:S894-5. [Crossref]
- Jänne PA, Rybkin II, Spira AI, et al. KRYSTAL-: Activity and Safety of Adagrasib (MRTX849) in Advanced/ Metastatic Non–Small-Cell Lung Cancer (NSCLC) Harboring KRASG12C Mutation. Eur J Cancer 2020;138:S1-2. [Crossref]
- A Phase Trial of MRTX849 in Combination with Pembrolizumab in Patients with Advanced Non Small Cell Lung Cancer with KRASG12C Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT04613596?term=mrtx849&draw=2&rank=1
- Hallin J, Engstrom LD, Hargis L, et al. The KRASG12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov 2020;10:54-71. [Crossref] [PubMed]
- Phase Study of MRTX849 vs Docetaxel in Patients with Advanced Non-Small Cell Lung Cancer With KRASG12C Mutation (KRYSTAL-12). Available online: https://clinicaltrials.gov/ct2/show/NCT04685135?term=mrtx849&draw=2&rank=3
- Awad MM, Liu S, Rybkin II, et al. Acquired Resistance to KRASG12C Inhibition in Cancer. N Engl J Med 2021;384:2382-93. [Crossref] [PubMed]
- Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer 2015;15:7-24. [Crossref] [PubMed]
- Beck JT, Ismail A, Tolomeo C. Targeting the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway: an emerging treatment strategy for squamous cell lung carcinoma. Cancer Treat Rev 2014;40:980-9. [Crossref] [PubMed]
- Li S, Li L, Zhu Y, et al. Coexistence of EGFR with KRAS, or BRAF, or PIK3CA somatic mutations in lung cancer: a comprehensive mutation profiling from 5125 Chinese cohorts. Br J Cancer 2014;110:2812-20. [Crossref] [PubMed]
- Sawa K, Koh Y, Kawaguchi T, et al. PIK3CA mutation as a distinctive genetic feature of non-small cell lung cancer with chronic obstructive pulmonary disease: A comprehensive mutational analysis from a multi-institutional cohort. Lung Cancer 2017;112:96-101. [Crossref] [PubMed]
- McGowan M, Hoven AS, Lund-Iversen M, et al. PIK3CA mutations as prognostic factor in squamous cell lung carcinoma. Lung Cancer 2017;103:52-7. [Crossref] [PubMed]
- Wang Y, Wang Y, Li J, et al. Clinical Significance of PIK3CA Gene in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Biomed Res Int 2020;2020:3608241. [Crossref] [PubMed]
- Scheffler M, Bos M, Gardizi M, et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget 2015;6:1315-26. [Crossref] [PubMed]
- Trejo CL, Green S, Marsh V, et al. Mutationally activated PIK3CA(H1047R) cooperates with BRAF(V600E) to promote lung cancer progression. Cancer Res 2013;73:6448-61. [Crossref] [PubMed]
- Spoerke JM, O'Brien C, Huw L, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res 2012;18:6771-83. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Soria JC, Adjei AA, Bahleda R, et al. A phase IB dose-escalation study of the safety and pharmacokinetics of pictilisib in combination with either paclitaxel and carboplatin (with or without bevacizumab) or pemetrexed and cisplatin (with or without bevacizumab) in patients with advanced non-small cell lung cancer. Eur J Cancer 2017;86:186-96. [Crossref] [PubMed]
- Levy B, Spira A, Becker D, et al. A randomized, phase trial of Docetaxel with or without PX‐866, an irreversible oral phosphatidylinositol 3‐kinase inhibitor, in patients with relapsed or metastatic non‐small‐cell lung cancer. J Thorac Oncol 2014;9:1031-5. [Crossref] [PubMed]
- Vansteenkiste JF, Canon JL, De Braud F, et al. Safety and Efficacy of Buparlisib (BKM120) in Patients with PI3K Pathway-Activated Non-Small Cell Lung Cancer: Results from the Phase II BASALT-1 Study. J Thorac Oncol 2015;10:1319-27. [Crossref] [PubMed]
- Adjei AA, Bennouna J, Leighl NB, et al. Safety and efficacy of buparlisib (BKMand chemotherapy in advanced, squamous non‐small cell lung cancer (sqNSCLC): Results from the phase Ib/II BASALT‐2 and BASALT‐3 studies. J Clin Oncol 2016;34:e20522. [Crossref]
- Langer CJ, Redman MW, Wade JL 3rd, et al. SWOG S1400B (NCT02785913), a Phase II Study of GDC-0032 (Taselisib) for Previously Treated PI3K-Positive Patients with Stage IV Squamous Cell Lung Cancer (Lung-MAP Sub-Study). J Thorac Oncol 2019;14:1839-46. [Crossref] [PubMed]
- Jhaveri K, Chang MT, Juric D, et al. Phase I Basket Study of Taselisib, an Isoform-Selective PI3K Inhibitor, in Patients with PIK3CA-Mutant Cancers. Clin Cancer Res 2021;27:447-59. [Crossref] [PubMed]
- Henderson IC, Spigel DR, Nemunaitis JJ, et al. A phase 1 study of weekly, divided dose perifosine in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2006;24:abstr 13063.
- Lara PN Jr, Longmate J, Mack PC, et al. Phase II Study of the AKT Inhibitor MK-2206 plus Erlotinib in Patients with Advanced Non-Small Cell Lung Cancer Who Previously Progressed on Erlotinib. Clin Cancer Res 2015;21:4321-6. [Crossref] [PubMed]
- Soria JC, Shepherd FA, Douillard JY, et al. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol 2009;20:1674-81. [Crossref] [PubMed]
- Vansteenkiste J, Solomon B, Boyer M, et al. Everolimus in combination with pemetrexed in patients with advanced non-small cell lung cancer previously treated with chemotherapy: a phase I study using a novel, adaptive Bayesian dose-escalation model. J Thorac Oncol 2011;6:2120-9. [Crossref] [PubMed]
- Besse B, Leighl N, Bennouna J, et al. Phase II study of everolimus-erlotinib in previously treated patients with advanced non-small-cell lung cancer. Ann Oncol 2014;25:409-15. [Crossref] [PubMed]
- Price KA, Azzoli CG, Krug LM, et al. Phase II trial of gefitinib and everolimus in advanced non-small cell lung cancer. J Thorac Oncol 2010;5:1623-9. [Crossref] [PubMed]
- Blumenthal GM, Ballas MS, Bernstein W, et al. A phase I/II trial of pemetrexed and sirolimus in advanced NSCLC. J Clin Oncol ;28:abstr 7600.
- Gandhi L, Besse B, Mazieres J, et al. MA02 Neratinib ± Temsirolimus in HER-2 ‐mutant lung cancers: An international, randomized phase II study. J Thorac Oncol 2017;12:S358-9. [Crossref]
- Krebs M, Spicer J, Steele N, et al. P02c‐003 TAX‐TORC: The novel combination of weekly paclitaxel and the dual mTORC1/2 inhibitor AZD2014 for the treatment of squamous NSCLC. J Thorac Oncol 2017;12:S1272-3. [Crossref]
- Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol 2009;21:177-84. [Crossref] [PubMed]
- Casalini P, Iorio MV, Galmozzi E, et al. Role of HER receptors family in development and differentiation. J Cell Physiol 2004;200:343-50. [Crossref] [PubMed]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006;7:505-16. [Crossref] [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [Crossref] [PubMed]
- Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [Crossref] [PubMed]
- Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer 2012;12:553-63. [Crossref] [PubMed]
- Klapper LN, Glathe S, Vaisman N, et al. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci U S A 1999;96:4995-5000. [Crossref] [PubMed]
- Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A 2010;107:7692-7. [Crossref] [PubMed]
- Alimandi M, Romano A, Curia MC, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995;10:1813-21. [PubMed]
- Waterman H, Alroy I, Strano S, et al. The C-terminus of the kinase-defective neuregulin receptor ErbB-3 confers mitogenic superiority and dictates endocytic routing. EMBO J 1999;18:3348-58. [Crossref] [PubMed]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem 2001;276:2841-51. [Crossref] [PubMed]
- Sheng Q, Liu X, Fleming E, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell 2010;17:298-310. [Crossref] [PubMed]
- Wilson TR, Lee DY, Berry L, et al. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 2011;20:158-72. [Crossref] [PubMed]
- Lyne JC, Melhem MF, Finley GG, et al. Tissue expression of neu differentiation factor/heregulin and its receptor complex in prostate cancer and its biologic effects on prostate cancer cells in vitro. Cancer J Sci Am 1997;3:21-30. [PubMed]
- Kim HG, Lee CK, Cho SM, et al. Neuregulin 1 up-regulates the expression of nicotinic acetylcholine receptors through the ErbB2/ErbB3-PI3K-MAPK signaling cascade in adult autonomic ganglion neurons. J Neurochem 2013;124:502-13. [Crossref] [PubMed]
- Nagasaka M, Ou SI. Neuregulin 1 Fusion-Positive NSCLC. J Thorac Oncol 2019;14:1354-9. [Crossref] [PubMed]
- Trombetta D, Graziano P, Scarpa A, et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by Phospho-ErbB3 expression. Oncotarget 2018;9:9661-71. [Crossref] [PubMed]
- Jonna S, Feldman RA, Swensen J, et al. Detection of NRG1 Gene Fusions in Solid Tumors. Clin Cancer Res 2019;25:4966-72. [Crossref] [PubMed]
- Drilon A, Somwar R, Mangatt BP, et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov 2018;8:686-95. [Crossref] [PubMed]
- Fernandez-Cuesta L, Thomas RK. Molecular Pathways: Targeting NRG1 Fusions in Lung Cancer. Clin Cancer Res 2015;21:1989-94. [Crossref] [PubMed]
- Huang J, Wang S, Lyu H, et al. The anti-erbB3 antibody MM-121/SAR256212 in combination with trastuzumab exerts potent antitumor activity against trastuzumab-resistant breast cancer cells. Mol Cancer 2013;12:134. [Crossref] [PubMed]
- Meetze K, Vincent S, Tyler S, et al. Neuregulin 1 expression is a predictive biomarker for response to AV-203, an ERBB3 inhibitory antibody, in human tumor models. Clin Cancer Res 2015;21:1106-14. [Crossref] [PubMed]
- Le Clorennec C, Bazin H, Dubreuil O, et al. Neuregulin 1 Allosterically Enhances the Antitumor Effects of the Noncompeting Anti-HER3 Antibody 9F7-F11 by Increasing Its Binding to HER3. Mol Cancer Ther 2017;16:1312-23. [Crossref] [PubMed]
- Duruisseaux M, Liu SV, Han JY, et al. NRGfusion-positive lung cancers: Clinicopathologic profile and treatment outcomes from a global multicenter registry. J Clin Oncol 2019;37:abstr 9081.
- Gay ND, Wang Y, Beadling C, et al. Durable Response to Afatinib in Lung Adenocarcinoma Harboring NRG1 Gene Fusions. J Thorac Oncol 2017;12:e107-10. [Crossref] [PubMed]
- Han JY, Lim KY, Kim JY, et al. P02c-006 EGFR and HER3 Inhibition - A Novel Therapy for Invasive Mucinous Non-Small Cell Lung Cancer Harboring an NRG1 Fusion Gene. J Thorac Oncol 2017;12:S1274-5. [Crossref]
- Schram AM, Drilon A, Macarulla Mercade T, et al. TiP - A phase II basket study of MCLA-128, a bispecific antibody targeting the HER3 pathway, in NRG1 fusion-positive advanced solid tumors. Ann Oncol. 2019;30:v317. [Crossref]
- Shigematsu H, Takahashi T, Nomura M, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res 2005;65:1642-6. [Crossref] [PubMed]
- Stephens P, Hunter C, Bignell G, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature 2004;431:525-6. [Crossref] [PubMed]
- Mazières J, Peters S, Lepage B, et al. Lung cancer that harbors an HER2 mutation: epidemiologic characteristics and therapeutic perspectives. J Clin Oncol 2013;31:1997-2003. [Crossref] [PubMed]
- Arcila ME, Chaft JE, Nafa K, et al. Prevalence, clinicopathologic associations, and molecular spectrum of ERBB2 (HER2) tyrosine kinase mutations in lung adenocarcinomas. Clin Cancer Res 2012;18:4910-8. [Crossref] [PubMed]
- Li C, Sun Y, Fang R, et al. Lung adenocarcinomas with HER2-activating mutations are associated with distinct clinical features and HER2/EGFR copy number gains. J Thorac Oncol 2012;7:85-9. [Crossref] [PubMed]
- Wistuba II. Molecular testing of non-small cell lung carcinoma biopsy and cytology specimens. Am Soc Clin Oncol Educ Book 2012;459-64. [Crossref] [PubMed]
- Wang SE, Narasanna A, Perez-Torres M, et al. HER2 kinase domain mutation results in constitutive phosphorylation and activation of HER2 and EGFR and resistance to EGFR tyrosine kinase inhibitors. Cancer Cell 2006;10:25-38. [Crossref] [PubMed]
- Ekman S. HER2: defining a Neu target in non-small-cell lung cancer. Ann Oncol 2019;30:353-5. [Crossref] [PubMed]
- Rakha EA, Miligy IM, Quinn CM, et al. Retrospective observational study of HER2 immunohistochemistry in borderline breast cancer patients undergoing neoadjuvant therapy, with an emphasis on Group 2 (HER2/CEP17 ratio ≥2.0, HER2 copy number <4.0 signals/cell) cases. Br J Cancer 2021;124:1836-42. [Crossref] [PubMed]
- Remon J, Hendriks LEL, Cardona AF, et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev 2020;90:102105. [Crossref] [PubMed]
- Baraibar I, Mezquita L, Gil-Bazo I, et al. Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC. Crit Rev Oncol Hematol 2020;148:102906. [Crossref] [PubMed]
- de Langen AJ, Jebbink M, Hashemi SMS, et al. Trastuzumab and paclitaxel in patients with EGFR mutated NSCLC that express HER2 after progression on EGFR TKI treatment. Br J Cancer 2018;119:558-64. [Crossref] [PubMed]
- Dziadziuszko R, Smit EF, Dafni U, et al. Afatinib in NSCLC With HER2 Mutations: Results of the Prospective, Open-Label Phase II NICHE Trial of European Thoracic Oncology Platform (ETOP). J Thorac Oncol 2019;14:1086-94. [Crossref] [PubMed]
- Lai WV, Lebas L, Barnes TA, et al. Afatinib in patients with metastatic or recurrent HER2-mutant lung cancers: a retrospective international multicentre study. Eur J Cancer 2019;109:28-35. [Crossref] [PubMed]
- Kris MG, Camidge DR, Giaccone G, et al. Targeting HER2 aberrations as actionable drivers in lung cancers: phase II trial of the pan-HER tyrosine kinase inhibitor dacomitinib in patients with HER2-mutant or amplified tumors. Ann Oncol 2015;26:1421-7. [Crossref] [PubMed]
- Besse B, Soria JC, Yao B, et al. Neratinib (N) with or Without Temsirolimus (Tem) in Patients (Pts) with Non-Small Cell Lung Cancer (Nsclc) Carrying HerSomatic Mutations: an International Randomized Phase Ii Study. Ann Oncol 2014;25:v1. [Crossref]
- Mazières J, Barlesi F, Filleron T, et al. Lung cancer patients with HER2 mutations treated with chemotherapy and HER2-targeted drugs: results from the European EUHER2 cohort. Ann Oncol 2016;27:281-6. [Crossref] [PubMed]
- Gonzalvez F, Zhu X, Huang WS, et al. AP, a potent, selective inhibitor of EGFR and HER-2 oncogenic mutants, including exon 20 insertions, in preclinical models. Cancer Res 2016;76:abstr 2644.
- Riely GJ, Neal JW, Camidge DR, et al. Activity and Safety of Mobocertinib (TAK-788) in Previously Treated Non-Small Cell Lung Cancer with EGFR Exon 20 Insertion Mutations from a Phase I/II Trial. Cancer Discov 2021;11:1688-99. [Crossref] [PubMed]
- Elamin YY, Robichaux JP, Carter BW, et al. Poziotinib for Patients With HER2 Exon 20 Mutant Non-Small-Cell Lung Cancer: Results From a Phase II Trial. J Clin Oncol 2021; [Epub ahead of print]. [PubMed]
- Socinski MA, Cornelissen R, Garassino MC, et al. ZENITH, a multinational, multi-cohort phase II study of poziotinib in NSCLC patients with EGFR or HER-2 exon 20 insertion mutations. Ann Oncol 2020;31:S1188. [Crossref]
- Wang Y, Jiang T, Qin Z, et al. HER2 exon 20 insertions in non-small-cell lung cancer are sensitive to the irreversible pan-HER receptor tyrosine kinase inhibitor pyrotinib. Ann Oncol 2019;30:447-55. [Crossref] [PubMed]
- Li BT, Shen R, Buonocore D, et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol 2018;36:2532-7. [Crossref] [PubMed]
- Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016;107:1039-46. [Crossref] [PubMed]
- Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol 2017;18:1512-22. [Crossref] [PubMed]
- Smit EF, Nakagawa K, Nagasaka M, et al. Trastuzumab deruxtecan (T-DXd; DS-in patients with HER2-mutated metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J Clin Oncol. 2020;38:abstr 9504.
- Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2021; [Epub ahead of print]. [PubMed]
- Estrada-Bernal A, Le AT, Doak AE, et al. Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin Cancer Res 2021;27:1463-75. [Crossref] [PubMed]
- Liu SV, Villaruz LC, Lee VHF, et al. First analysis of RAIN-: Study of tarloxotinib in patients with non-small cell lung cancer (NSCLC) EGFR Exon 20 insertion, HER2-activating mutations & other solid tumours with NRG1/ERBB-gene fusions. Ann Oncol 2020;31:S1189. [Crossref]
- Wang R, Wang L, Li Y, et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non-small cell lung cancer. Clin Cancer Res 2014;20:4107-14. [Crossref] [PubMed]
- Qin A, Johnson A, Ross JS, et al. Detection of Known and Novel FGFR Fusions in Non-Small Cell Lung Cancer by Comprehensive Genomic Profiling. J Thorac Oncol 2019;14:54-62. [Crossref] [PubMed]
- Li JJ, Yan S, Pan Y, et al. FGFR genes mutation is an independent prognostic factor and associated with lymph node metastasis in squamous non-small cell lung cancer. Cancer Biol Ther 2018;19:1108-16. [Crossref] [PubMed]
- Lim SM, Kim HR, Shim HS, et al. Role of FGF receptors as an emerging therapeutic target in lung squamous cell carcinoma. Future Oncol 2013;9:377-86. [Crossref] [PubMed]
- Weiss J, Sos ML, Seidel D, et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010;2:62ra93. [Crossref] [PubMed]
- Helsten T, Elkin S, Arthur E, et al. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res 2016;22:259-67. [Crossref] [PubMed]
- Hibi M, Kaneda H, Tanizaki J, et al. FGFR gene alterations in lung squamous cell carcinoma are potential targets for the multikinase inhibitor nintedanib. Cancer Sci 2016;107:1667-76. [Crossref] [PubMed]
- ODonnell P. A Phase I Dose-escalation Study of MFGRS, a Human Monoclonal Anti-fibroblast Growth Factor Receptor 3 (FGFR3) Antibody, in Patients (pts) with Advanced Solid Tumors. Eur J Cancer 2012;191-2.
- Ren M, Hong M, Liu G, et al. Novel FGFR inhibitor ponatinib suppresses the growth of non-small cell lung cancer cells overexpressing FGFR1. Oncol Rep 2013;29:2181-90. [Crossref] [PubMed]
- Lim SH, Sun JM, Choi YL, et al. Efficacy and safety of dovitinib in pretreated patients with advanced squamous non-small cell lung cancer with FGFR1 amplification: A single-arm, phase 2 study. Cancer 2016;122:3024-31. [Crossref] [PubMed]
- Morgensztern D, Karaseva N, Felip E, et al. An open-label phase IB study to evaluate GSK3052230 in combination with paclitaxel and carboplatin, or docetaxel, in FGFR1-amplified non-small cell lung cancer. Lung Cancer 2019;136:74-9. [Crossref] [PubMed]
- Aggarwal C, Redman MW, Lara PN Jr, et al. SWOG S1400D (NCT02965378), a Phase II Study of the Fibroblast Growth Factor Receptor Inhibitor AZD4547 in Previously Treated Patients With Fibroblast Growth Factor Pathway-Activated Stage IV Squamous Cell Lung Cancer (Lung-MAP Substudy). J Thorac Oncol 2019;14:1847-52. [Crossref] [PubMed]
- Nogova L, Malchers F, Hillmer A, et al. FIND: A phase II study to evaluate the efficacy of erdafitinib in FGFR-altered squamous NSCLC. Ann Oncol ;30:ii67. [Crossref]
- Subbiah V, Barve M, Iannotti NO, et al. FIGHT-: A phase 1/2 study of pemigatinib, a highly selective fibroblast growth factor receptor (FGFR) inhibitor, as monotherapy and as combination therapy in patients with advanced malignancies. Mol Cancer Ther 2019;18:abstr A078.
- Haslam A, Kim MS, Prasad V. Updated estimates of eligibility for and response to genome-targeted oncology drugs among US cancer patients, 2006-2020. Ann Oncol 2021;32:926-32. [Crossref] [PubMed]
- Peters S, Mok T, Passaro A, et al. The Promising Evolution of Targeted Therapeutic Strategies in Cancer. Cancer Discov 2021;11:810-4. [Crossref] [PubMed]
- Britschgi C, Addeo A, Rechsteiner M, et al. Real-World Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small-Cell Lung Cancer Patients. Front Oncol 2020;10:1299. [Crossref] [PubMed]
- Fois SS, Paliogiannis P, Zinellu A, et al. Molecular Epidemiology of the Main Druggable Genetic Alterations in Non-Small Cell Lung Cancer. Int J Mol Sci 2021;22:612. [Crossref] [PubMed]
- Kimmelman J, Tannock I. The paradox of precision medicine. Nat Rev Clin Oncol 2018;15:341-2. [Crossref] [PubMed]
饶欣欣
复旦大学附属肿瘤医院(更新时间:2023-05-05)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Filetti M, Rossi A, Taurelli Salimbeni B, Piras M, Rogges E, Napoli AD, Marchetti P, Giusti R. New driver alterations in non-small cell lung cancer: a narrative review. Precis Cancer Med 2022;5:5.

