
Squeezed into defection?—nuclear displacement by steatosis activates yes-associated protein (YAP) linked to oncogenic pathways in hepatocytes
Nonalcoholic fatty liver disease (NAFLD) has become the most prevalent liver disorder of our times, estimated to affect at least a quarter of the world’s population (1). Histologically, NAFLD ranges from steatosis to steatohepatitis with an increasing risk to progress into cirrhosis and hepatocellular carcinoma (2). Most cases of NAFLD-associated HCC develop in cirrhosis, but HCC may complicate non-cirrhotic disease, representing a major logistical challenge as our current understanding of the drivers of liver tumorigenesis at early stages of NAFLD remains insufficient to guide risk assessment and personalized prediction (3).
Genetic aberrations promoting the onset and progression of HCC are notoriously diverse, making screening, treatment and prognostication difficult (4,5). Cellular and molecular mechanisms that contribute to the development of HCC in NAFLD involve DNA damage responses, inflammatory changes and interactions with the gut microbiome (6). Recent research indicates that various developmental pathways including Notch, Wnt/beta-catenin, Hedgehog and Hippo/yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) substantially contribute to this process (7). In advanced liver disease, these regulatory cascades are chronically activated and create a microenvironment conducive to uncontrolled hepatocellular growth and genomic instability, consistent with a high risk of HCC in cirrhosis (7). While altered hepatocellular lipid metabolism, insulin resistance and some other mechanisms have been implicated in the development of non-cirrhotic HCC, much less is known about liver tumorigenesis in the absence of significant inflammation and fibrosis (8,9).
One of the many molecular signaling cascades implicated in HCC development, Hippo/YAP/TAZ is an evolutionarily conserved pathway that regulates organ development and regeneration, acting through multiple intracellular kinases to control the activity of YAP and the paralog TAZ, which may otherwise translocate to the nucleus and induce the expression of genes involved in cell growth and proliferation (10,11). Activation of YAP/TAZ as a gene regulatory tool is shared by several developmental pathways including G protein-coupled receptors and the transforming growth factor-β (TGFβ) and Wnt/β-catenin cascades (12). In the liver, YAP activation has been linked to hepatocyte growth factor/MET signaling and glypican 3-mediated tumorigenesis (13,14). Sustained activation of YAP/TAZ is therefore a major oncogenic force identified in many tissues including the liver (15,16).
Hippo/YAP/TAZ signaling has a key role in mechanotransduction, which is an overarching term for the cellular responses to physical stimuli that are translated into biochemical signals (17). Mechanical stress in biological tissues may result from shear, stretch and compression forces that activate a variety of cellular mechanosensors found in the cytoskeleton (actin, microtubules, and intermediate filaments) and on the cell surface where integrins, focal adhesions and ion channels detect conformational changes related to cell-cell and cell-substrate interactions (18). Rather than being committed to a specific extracellular ligand/receptor, YAP/TAZ is activated in response to many of these physical signals relevant to cell-cell contact and positional changes (17,19,20). Sustained activation of YAP/TAZ has been described in parenchymal hepatocytes, liver sinusoidal endothelial cells (LSECs), Kupffer cells and hepatic stellate cells (HSCs) in response to increased tissue stiffness due to inflammation, fibrosis or elevated sinusoidal pressure (21-24). However, we know little about the potential role of intrahepatic lipid accumulation in generating mechanocrine signals in NAFLD.
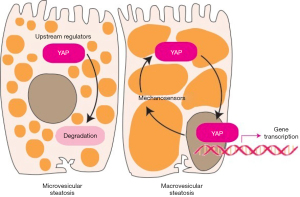
In a recent work, Chin et al. (25) focused on the earliest steps of NAFLD pathophysiology to investigate the impact of steatosis on YAP signaling, based on the hypothesis that lipid droplets in hepatocytes may disrupt mechanosensing, similar to what we see in response to increased stiffness due to fibrosis or inflammation. To answer this intriguing question, the authors first analyzed if there is a correlation between tissue stiffness and lipid content in liver specimens obtained from patients who underwent liver resection or transplantation with NAFLD-associated and non-NAFLD cirrhosis. By using parallel plate rheometer, they found that neither the etiology of cirrhosis nor the amount of intrahepatic lipid affected liver tissue stiffness (25). This is not very surprising since stiffness in cirrhosis is predominantly determined by an excessive degree of fibrosis, and the presence of intracellular lipids (which is often diminishing in NAFLD-cirrhosis anyway) may not have a significant additional impact (26). Further analysis of liver specimens derived from NAFLD-cirrhosis revealed that individual hepatocytes have heterogeneous distribution of intracellular fat, consisting of cells with large lipid droplets displacing the nucleus and cells with small lipid droplets and their nucleus left in place. When intracellular distribution of YAP was analyzed, the relative number of cells with YAP-positive nuclei positively correlated with the size of lipid droplets, indicating that YAP translocation was more likely to occur in nuclei squeezed to the side of hepatocytes (25).
To further analyze the impact of lipid droplets on hepatocellular mechanics, Chin et al. loaded human HCC-derived Huh-7 cells and primary human hepatocytes with oleic acid and linoleic acid at doses not too high to cause cytotoxicity, senescence or apoptosis (25). They then investigated the spreading of lipid-loaded cells on substrates with increasing stiffness, which showed inverse correlation with the amount of intracellular lipids. Moreover, they demonstrated that fatty acid treatment had an adverse effect on focal adhesions and stress fibers, indicating disruption of the cytoskeleton. While the proportion of YAP-positive nuclei did not correlate with substrate stiffness, it increased significantly in response to the addition of insulin (promoting the formation of large lipid droplets), indicating that nuclear displacement has a positive impact on YAP translocation (25). These findings suggest that large lipid droplets and nuclear displacement, frequently observed in macrovesicular steatosis, could become a source of mechanical stress in hepatocytes and promote YAP activation, thus establishing a novel mechanistic link between hepatocellular lipid accumulation and oncogenesis.
It is somewhat surprising that YAP translocation was not affected by substrate stiffness in primary cultured hepatocytes and Huh-7 cells, although it was stimulated by formation of large lipid droplets, disruption of the cytoskeleton, and displacement of the nucleus. This may perhaps indicate that YAP responds more readily to mechanocrine signals emanating from the cytoskeleton than from cell surface mechanosensors, at least in this experimental setting. Specific transcriptional targets of YAP were not looked at by Chin and colleagues, but the broad impact of YAP/TAZ activation is likely to involve cancer-promoting effects when these events occur in vivo (16). Notably, while YAP translocation was essentially universal in hepatocytes with large lipid droplets identified in NAFLD-cirrhosis specimens, the proportion of YAP-positive nuclei still reached about 60% in hepatocytes with small lipid droplets or no steatosis at all (25). This indicates that, regardless of the presence or size of lipid droplets, YAP signaling is mostly active in cirrhosis, while persisting steatosis may further enhance YAP activation.
Intriguingly, the association between YAP-positive nuclei and large lipid droplets in human liver samples analyzed by Chin and colleagues is seemingly at variance with a recent report from Japan, in which 154 HCC patients were histologically classified based on the extent of tumor tissue steatosis, and the presence of large lipid droplets predicted better survival (27). Although distribution of YAP was not analyzed in this work, YAP activation, if it occurs in macro-steatotic HCC, is unlikely to have a beneficial effect on clinical outcomes. Of note, YAP and TAZ have rather heterogeneous localization patterns and co-expression profiles in HCC, and the prognostic significance of cytoplasmic and nuclear presence of YAP/TAZ remains to be determined (28).
NAFLD is a highly heterogeneous disease and steatosis has been generally associated with benign liver outcomes. Lipids are primarily stored in hepatocytes as triglycerides, which have been considered relatively harmless with no direct correlation to the amount of lipid derivatives metabolites that drive lipotoxicity in hepatocytes (29). While several questions remain unanswered, the work of Chen and colleagues represents a provocative novel paradigm of how mechanosignaling originating from the physical impact of lipid droplets may potentially contribute to the activation of oncogenic pathways in early-stage NAFLD (Figure 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at: https://dx.doi.org/10.21037/pcm-21-21). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Younossi ZM. Non-alcoholic fatty liver disease - A global public health perspective. J Hepatol 2019;70:531-44. [Crossref] [PubMed]
- Powell EE, Wong VW, Rinella M. Non-alcoholic fatty liver disease. Lancet 2021;397:2212-24. [Crossref] [PubMed]
- Loomba R, Lim JK, Patton H, et al. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020;158:1822-30. [Crossref] [PubMed]
- Craig AJ, von Felden J, Garcia-Lezana T, et al. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2020;17:139-52. [Crossref] [PubMed]
- Barcena-Varela M, Lujambio A. The Endless Sources of Hepatocellular Carcinoma Heterogeneity. Cancers (Basel) 2021;13:2621. [Crossref] [PubMed]
- Anstee QM, Reeves HL, Kotsiliti E, et al. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 2019;16:411-28. [Crossref] [PubMed]
- Zhu C, Tabas I, Schwabe RF, et al. Maladaptive regeneration - the reawakening of developmental pathways in NASH and fibrosis. Nat Rev Gastroenterol Hepatol 2021;18:131-42. [Crossref] [PubMed]
- Baffy G. Hepatocellular Carcinoma in Obesity: Finding a Needle in the Haystack? Adv Exp Med Biol 2018;1061:63-77. [Crossref] [PubMed]
- Geh D, Anstee QM, Reeves HL. NAFLD-Associated HCC: Progress and Opportunities. J Hepatocell Carcinoma 2021;8:223-39. [Crossref] [PubMed]
- Patel SH, Camargo FD, Yimlamai D. Hippo Signaling in the Liver Regulates Organ Size, Cell Fate, and Carcinogenesis. Gastroenterology 2017;152:533-45. [Crossref] [PubMed]
- Werneburg N, Gores GJ, Smoot RL. The Hippo Pathway and YAP Signaling: Emerging Concepts in Regulation, Signaling, and Experimental Targeting Strategies With Implications for Hepatobiliary Malignancies. Gene Expr 2020;20:67-74. [Crossref] [PubMed]
- Dobrokhotov O, Samsonov M, Sokabe M, et al. Mechanoregulation and pathology of YAP/TAZ via Hippo and non-Hippo mechanisms. Clin Transl Med 2018;7:23. [Crossref] [PubMed]
- Feng M, Gao W, Wang R, et al. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc Natl Acad Sci U S A 2013;110:E1083-91. [Crossref] [PubMed]
- Xue Y, Bhushan B, Mars WM, et al. Phosphorylated Ezrin (Thr567) Regulates Hippo Pathway and Yes-Associated Protein (Yap) in Liver. Am J Pathol 2020;190:1427-37. [Crossref] [PubMed]
- Zhang S, Zhou D. Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol 2019;61:64-71. [Crossref] [PubMed]
- Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016;29:783-803. [Crossref] [PubMed]
- Kang N. Mechanotransduction in Liver Diseases. Semin Liver Dis 2020;40:84-90. [Crossref] [PubMed]
- Ohashi K, Fujiwara S, Mizuno K. Roles of the cytoskeleton, cell adhesion and rho signalling in mechanosensing and mechanotransduction. J Biochem 2017;161:245-54. [Crossref] [PubMed]
- Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle 2012;11:1090-6. [Crossref] [PubMed]
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015;15:73-9. [Crossref] [PubMed]
- Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol 2015;63:679-88. [Crossref] [PubMed]
- Song Z, Gupta K, Ng IC, et al. Mechanosensing in liver regeneration. Semin Cell Dev Biol 2017;71:153-67. [Crossref] [PubMed]
- Noguchi S, Saito A, Nagase T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int J Mol Sci 2018;19:3674. [Crossref] [PubMed]
- Song K, Kwon H, Han C, et al. Yes-Associated Protein in Kupffer Cells Enhances the Production of Proinflammatory Cytokines and Promotes the Development of Nonalcoholic Steatohepatitis. Hepatology 2020;72:72-87. [Crossref] [PubMed]
- Chin L, Theise ND, Loneker AE, et al. Lipid droplets disrupt mechanosensing in human hepatocytes. Am J Physiol Gastrointest Liver Physiol 2020;319:G11-22. [Crossref] [PubMed]
- Nagula S, Jain D, Groszmann RJ, et al. Histological-hemodynamic correlation in cirrhosis-a histological classification of the severity of cirrhosis. J Hepatol 2006;44:111-7. [Crossref] [PubMed]
- Kubota N, Ojima H, Hatano M, et al. Clinicopathological features of hepatocellular carcinoma with fatty change: Tumors with macrovesicular steatosis have better prognosis and aberrant expression patterns of perilipin and adipophilin. Pathol Int 2020;70:199-209. [Crossref] [PubMed]
- Van Haele M, Moya IM, Karaman R, et al. YAP and TAZ Heterogeneity in Primary Liver Cancer: An Analysis of Its Prognostic and Diagnostic Role. Int J Mol Sci 2019;20:638. [Crossref] [PubMed]
- Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev Gastroenterol Hepatol 2009;3:445-51. [Crossref] [PubMed]
Cite this article as: Baffy G. Squeezed into defection?—nuclear displacement by steatosis activates yes-associated protein (YAP) linked to oncogenic pathways in hepatocytes. Precis Cancer Med 2021;4:40.

