
Partial hippocampal sparing whole brain radiotherapy in a patient with bilateral malignant melanoma metastases to the hippocampus: a case report
Introduction
Whole brain radiotherapy (WBRT) has been used for decades in the treatment of metastatic cancer to the brain. It is a commonly used palliative technique: around 200,000 patients receive WBRT each year in the treatment of brain metastases, in the United States alone (1). In unselected cases, there is an evidence that WBRT extends survival over best supportive care; WBRT can regress brain metastases and can prevent brain metastasis-associated neurological decline, and in particular, improve radiological intracranial disease control (1-4).
However, not all clinical trials have demonstrated clear-cut clinical benefits of WBRT, and omission of WBRT after stereotactic radiosurgery (SRS) or surgery does not impair overall survival (5). Additionally, WBRT is associated with neurocognitive and quality-of-life deficits, especially evident using the sensitive measurement instruments of the modern era (6,7).
Hippocampal-avoidance WBRT (HA-WBRT) has become a more prevalent radiotherapy technique in recent years. Using volumetric modulated arc therapy (VMAT) (8), the region around the hippocampus can be selectively excluded from the higher dose delivered to the rest of the brain. The rationale for the technique is that the hippocampus contributes to higher neurocognitive function, especially memory and learning, and its irradiation during WBRT can be a cause of neurocognitive decline. In fact, preclinical studies have shown dysfunction of hippocampal neural progenitor cells with radiation doses as low as 2 Gy (9,10). Hippocampal avoidance was hence developed to attempt to circumvent some of the neurocognitive decline associated with WBRT (11).
We report a case where HA-WBRT was delivered to a patient with multiple brain metastases, including involvement of bilateral hippocampi. In this case, we avoided the WBRT dose to the remaining non-tumour-affected regions of the hippocampi. To our knowledge, it is the only case reporting partial hippocampal avoidance in HA-WBRT.
We present the following case in accordance with the CARE reporting checklist (available at https://dx.doi.org/10.21037/pcm-21-23).
Case presentation
A previously well 64-year-old male presented to our hospital with four days of vertigo, nausea, vomiting and headache. MRI brain showed two masses with the appearance of haemorrhagic metastases: one in the right frontal lobe (5 mm maximum dimension), one in the right cerebellar hemisphere/middle cerebellar peduncle (21 mm maximum dimension). Both lesions showed post-contrast enhancement. There was mild effacement of the fourth ventricle, but no obstructive hydrocephalus. The patient’s clinical condition improved on dexamethasone 8 mg tds. Because the brain lesions appeared metastatic, staging included a CT scan of the chest, abdomen and pelvis and an FDG PET/CT scan; these investigations showed a large left lower lung mass and mediastinal lymphadenopathy. A provisional diagnosis of metastatic lung cancer was made, and bronchoscopy and endobronchial ultrasound (EBUS) biopsy of the mediastinal nodes was performed. This yielded lymphoid tissue alone, and no endobronchial lesion was seen. Histomorphology on subsequent CT-guided core biopsy of the lung mass showed tumour comprised of sheaths of large atypical cells with a moderate amount of eosinophilic cytoplasm and indistinct cell borders. Immunohistologically, the tumour cells showed positive staining for SOX-10 and Melan-A. There was negative staining for cytokeratin AE1/AE3 and S-100. The overall histopathological features were of metastatic malignant melanoma. Tumour tissue harboured an NRAS, but not BRAF, mutation.
The patient gave a retrospective history of removal of a non-pigmented skin lesion, approximately 10 years earlier. He had no history of malignant melanoma or removal of other skin lesions, and comprehensive dermal evaluation revealed no primary tumour site.
The patient was ineligible for targeted therapy because of the BRAF-wildtype status of his tumour. He was commenced on immunotherapy and at the time of writing, had received three cycles of pembrolizumab, with stable disease extracranially, but with intracranial progression.
The patient next underwent SRS (this refers to a single fraction of focussed cerebral radiotherapy; traditionally, more than one fraction is referred to as stereotactic radiotherapy, but for simplicity, we refer to both here as SRS) to the two cerebral lesions. The right frontal lesion received 21.2 Gy in a single fraction, whereas the cerebellar lesion, because of its size and proximity to the brainstem, was treated with 31.8 Gy in 3×10.6 Gy fractions. The procedure was well tolerated.
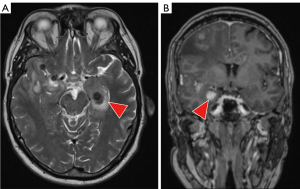
Eight weeks later and 9 weeks after the third cycle of pembrolizumab, he developed progressive headaches, nausea and vomiting, and a further brain MRI scan showed 14 space-occupying lesions, and palliative WBRT was contemplated. Because of his relatively young age and good performance status, HA-WBRT was considered; however, he had metastases in the hippocampi bilaterally, which could be considered a contraindication to HA-WBRT (Figure 1). Both lesions were in the anterior part of the hippocampi. The patient was not commenced on memantine, but underwent radiotherapy planning for HA-WBRT, with the modification that the two hippocampal metastases were included in the higher dose volume, and were carefully delineated from the uninvolved hippocampi, which were in the radiation avoidance region (Figure 2).
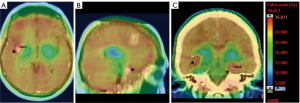
For the right hippocampal avoidance structure (blue-green in Figure 2), the D100% was 10 Gy, maximum 18.4 Gy, while for the left hippocampal avoidance structure (blue-green in Figure 2), the D100% was 8.45 Gy, maximum 18.1 Gy. The planning target volume (PTV) received 28.9 Gy, with <2% receiving >31 Gy. We prioritised minimising hot spots in the PTV because of the patient’s previous SRS treatment.
The patient remains fit and well with no gross memory or cognition issues, 14 days after completing partial hippocampal sparing WBRT. No formal quality of life studies were performed.
All procedures were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Here we report a rare case of partial HA-WBRT in a patient with multiple metastatic malignant melanoma metastases to the brain, including bilateral hippocampal lesions. The rationale for the partial HA-WBRT was to spare as much of the uninvolved hippocampi as possible, while still treating the hippocampal metastases, along with the rest of the brain. It is unknown in this case what the neurocognitive outcomes were. To our knowledge, partial HA-WBRT has not been reported before, but could be worth considering when HA-WBT is being entertained and the patient has hippocampal metastases.
Although it is not the only part of the brain that does so, the hippocampus subserves higher cognitive functions, including short-term memory and learning (12). Since the HA-WBRT technique appears safe (i.e., not associated with overt metastatic recurrence within hippocampal proximity) (11), it is an attractive approach. This approach was investigated in the RTOG 0933 phase II single arm trial of HA-WBRT in patients with brain metastases from epithelial malignancies (11). These investigators found lower rates of recall decline when HA-WBRT was used, compared to conventionally-treated WBRT historical controls. However, some concerns were raised that the historical controls differed from those receiving HA-WBRT, for example, by having a lower median survival. Furthermore, the entire approach has been questioned on the basis that cognitive function is not entirely a hippocampal function, so sparing the hippocampus may have unknown benefits (13). To clarify the potential utility of HA-WBRT, further (some randomised) clinical trials of HA-WBRT are in progress (at least 11 recruiting trials and eight of unknown status as at June 2021) (Clinicaltrials.gov).
The frequency of hippocampal metastases among brain metastasis patients is 8% (14). As far as we know, the frequency of bilateral hippocampal metastases in brain metastasis patients, as reported in our case, is not known. At the least, it is rare.
Anterior and posterior parts of the hippocampus have likely different functions: the posterior hippocampus has been ascribed functions of spatial navigation and memory, while the anterior hippocampus mediates anxiety-associated behaviour, although this may be somewhat of an oversimplification (15). The location of hippocampal metastases and radiation doses across the hippocampi may have implications for the different hippocampal functions. Where possible, minimising irradiation to even one hippocampus might prove beneficial. Post-irradiation hippocampal atrophy is associated with degraded neurological function, but after low radiation doses a compensatory hippocampal increase in volume has been observed (16); these authors suggested that delivering the lowest dose to one hippocampus might help preserve cognition. Finally, in the case described here, the hippocampal metastases received the whole brain dose. Conceivably, dose-escalating these metastases using SRS may have further improved local control and outcome.
In summary, ongoing clinical trials are investigating the worth of HA-WBRT (17). Partial HA-WBRT, as described here, may be worth considering for HA-WBRT patients who harbour hippocampal metastases (18).
Written consent for the publication of deidentified information in this case report has been obtained from the relevant patient.
Acknowledgments
The authors thank Dr. Richard Foster for thoughtful comments on the manuscript and Hannah Martin for excellent technical assistance.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://dx.doi.org/10.21037/pcm-21-23
Peer Review File: Available at https://dx.doi.org/10.21037/pcm-21-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/pcm-21-23). MJM serves as an unpaid editorial board member of Precision Cancer Medicine from December 2019 to November 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brown PD, Ahluwalia MS, Khan OH, et al. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J Clin Oncol 2018;36:483-91. [Crossref] [PubMed]
- Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer 1954;7:682-9. [Crossref] [PubMed]
- Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol 2006;24:1295-304. [Crossref] [PubMed]
- Pinkham MB, Sahgal A, Pullar AP, et al. In response to Fogarty et al. and why adjuvant whole brain radiotherapy is not recommended routinely. BMC Cancer 2017;17:768. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol 2009;10:1037-44. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Teoh M, Clark CH, Wood K, et al. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 2011;84:967-96. [Crossref] [PubMed]
- Monje ML, Mizumatsu S, Fike JR, et al. Irradiation induces neural precursor-cell dysfunction. Nat Med 2002;8:955-62. [Crossref] [PubMed]
- Bálentová S, Hajtmanová E, Filova B, et al. Effect of Fractionated Irradiation on the Hippocampus in an Experimental Model. Klin Onkol 2015;28:191-9. [Crossref] [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Opitz B. Memory function and the hippocampus. Front Neurol Neurosci 2014;34:51-9. [Crossref] [PubMed]
- Aizer AA, Lee EQ. Brain Metastases. Neurol Clin 2018;36:557-77. [Crossref] [PubMed]
- Kazda T, Misove A, Burkon P, et al. Incidence of Hippocampal Metastases: Laterality and Implications for Unilateral Hippocampal Avoiding Whole Brain Radiotherapy. Biomed Res Int 2018;2018:2459608 [Crossref] [PubMed]
- Strange BA, Witter MP, Lein ES, et al. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014;15:655-69. [Crossref] [PubMed]
- Le Fèvre C, Cheng X, Loit MP, et al. Role of hippocampal location and radiation dose in glioblastoma patients with hippocampal atrophy. Radiat Oncol 2021;16:112. [Crossref] [PubMed]
- Brown PD, Gondi V, Pugh S, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J Clin Oncol 2020;38:1019-29. [Crossref] [PubMed]
- Hartgerink D, Swinnen A, Roberge D, et al. LINAC based stereotactic radiosurgery for multiple brain metastases: guidance for clinical implementation. Acta Oncol 2019;58:1275-82. [Crossref] [PubMed]
Cite this article as: McKay MJ, Zadeh HS, Sanderson C, McKay TA, Chindewere A. Partial hippocampal sparing whole brain radiotherapy in a patient with bilateral malignant melanoma metastases to the hippocampus: a case report. Precis Cancer Med 2021;4:39.

