
A narrative review of predictive and prognostic biomarkers in skin tumors
Introduction
Merkel cell carcinoma (MCC), melanoma, and keratinocyte carcinomas (KC), basal cell carcinoma (BCC) and squamous cell carcinoma, are among the most common and/or aggressive malignancies of the skin. Traditional staging parameters remain the most important and widely utilized prognostic and predictive tools. However, given the recent explosion in the literature on immunohistochemical and molecular-genetic markers for these skin tumors, novel predictive and prognostic biomarkers are emerging. Herein, we sought to summarize well-established and emerging predictive and prognostic biomarkers in these cutaneous malignancies.
We present the following article in accordance with the Narrative Review Reporting Checklist (available at: http://dx.doi.org/10.21037/pcm-21-6).
Methods
A systematic literature search was conducted in PubMed using the terms: Merkel cell carcinoma, melanoma, basal cell carcinoma, squamous cell carcinoma, keratinocyte carcinoma, staging, biomarker, and prognosis, over a 15-year interval from 2006 through 2021. Only English language articles were included. The American Joint Commission on Cancer (AJCC) staging criteria were also reviewed.
Non-melanoma skin cancers (NMSC)
MCC
Background
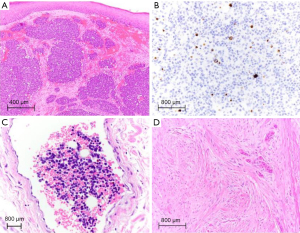
MCC is a clinically aggressive high-grade primary cutaneous neuroendocrine carcinoma with a rising incidence (1). MCC histopathologically demonstrates characteristic small round blue cells with scant cytoplasm, vesicular nuclei with fine, granular chromatin (‘salt and pepper’), multiple nucleoli, and frequent peri-nuclear dot-like immunoreactivity for cytokeratins (Figure 1A,1B). In tumors with demonstrated neuroendocrine differentiation (e.g., expression for synaptophysin, chromogranin, and CD56), a peri-nuclear pattern of positive staining for cytokeratin 20 (CK20) in conjunction with negative staining for thyroid transcription factor 1 (TTF-1) is highly specific for the diagnosis of MCC (2). This immunohistochemical profile is helpful for differentiating MCC from, for example, metastatic small-cell neuroendocrine carcinoma of the lung, in the vast majority of cases, but does not serve as a marker for prognostication or predict therapeutic response (3). Of note, CK20 negative MCC and MCC with TTF-1 and/or cytokeratin 7 expression have been reported (3). Despite the name suggestive of origin from epidermal Merkel cells, MCC predominantly involves the dermis and may demonstrate extension into the subcutis or involvement of the overlying epidermis (1). The cell of origin of MCC remains undefined (3).
Etiopathogenesis
MCC typically affects immunosuppressed and elderly individuals, particularly older Caucasian males, and commonly arises at sun-exposed skin sites such as those of the head and neck region (1). Indeed, chronic exposure to ultraviolet (UV) light mediates carcinogenesis in MCC (3). However, MCC etiopathogenesis is dichotomous. In a seminal study in 2008, Feng and colleagues utilized digital transcriptome subtraction and identified the novel Merkel cell polyomavirus (MCV or MCPyV) in the majority of human MCC tumors studied (80% of cases vs. 11% of non-MCC control tissues including skin) and demonstrated viral DNA integration into the tumor genome, suggestive of an etiopathogenic role of MCPyV in MCC tumorigenesis (4). MCC is now broadly divided into two subtypes: MCPyV- and MCPyV+. MCPyV- MCC demonstrates a high frequency of DNA mutations related to UV damage, aneuploidy, and inactivating mutations in various signaling pathways, including RB1 (RB transcriptional corepressor I which encodes the retinoblastoma-associated protein) and TP53 (tumor protein 53) (3). In contrast, MCPyV+ MCC lacks a UV mutational signature with wild type RB1 and TP53 and is predominantly diploid with infrequent copy number alterations (3). Cellular transformation in MCPyV+ MCC involves the expression of large T antigen (LT-ag) and small T antigen (ST-ag) genes (3). Interestingly, while the molecular function(s) of mutant ST is not well established, mutant LT is truncated with loss of DNA binding, helicase and cell growth-inhibitory domains but with preservation of the RB1-binding motif, LXCXE.
AJCC staging
MCC is an aggressive cutaneous malignancy with a 5-year overall survival rate of 55.6% for local disease, 35.4% for regional disease, and 13.5% for distant metastases (5). These data are based on the analysis of 9,387 patients from the National Cancer Database diagnosed with MCC between 1998 and 2012 comprising the eighth edition of the AJCC tumor node metastasis (TNM) staging classification for MCC (6,7).
The AJCC TNM staging criteria for MCC is used to define prognostic groups based on clinical and histopathological tumor parameters. Local disease is defined as primary MCC with clinical measurement of tumor size (T) as <2 cm (T1) (Stage I), >2 cm but <5 cm (T2), or >5 cm in greatest dimension (T3), or extension beyond the subcutis involving fascia, muscle, cartilage or bone (T4) (Stage II), without clinical or pathologic evidence of regional or distant metastases. Five-year overall survival rates vary from 55.8% for pT1, to 41.1% for pT2/pT3, and 31.8% for pT4 (2,7). Of note, the greatest dimension of the tumor may be represented by tumor diameter and may not be synonymous with tumor thickness (the maximum depth of invasion as measured in a perpendicular fashion from the epidermal granular cell layer).
Regional disease (Stage III) is stratified by clinical/radiologic and pathologic node (N) criteria (clinically detected regional lymph node metastasis (cN1), or clinically occult lymph node metastasis on sentinel lymph node biopsy with absence or presence of completion lymph node dissection (pN1a, pN1b, respectively), and encompasses in-transit metastases without lymph node metastasis (cN2, pN2) or both in-transit and lymph node metastasis (cN3, pN3). Of note, the eighth edition AJCC staging system for MCC no longer formally distinguishes the extent of intranodal tumor burden as ‘micrometastasis’ or ‘macrometastasis’ upon pathological evaluation (8), although Stage III patients with ‘non-solid’/non-diffuse sentinel lymph node involvement (Figure 1B) had improved overall survival (2). The five-year overall survival rate for clinically or radiologically detected and pathologically confirmed regional lymph node metastasis with unknown primary is 42.2% and drops to 26.8% in patients with documented primary MCC (5). Clinically detected lymph node metastasis portends a worse prognosis as compared to pathologically confirmed lymph node metastasis (7).
Distant metastases (M1), microscopically confirmed (Stage IV), are subcategorized by location: skin/subcutaneous (M1a), lung (M1b), and other locations (M1c) (5,6,8).
Biomarkers
A number of prognostic and predictive biomarkers in MCC have been studied. Among these are established markers of poor prognosis, such as advanced age, male gender, greater tumor depth, robust tumor-associated immune infiltrates, lymph node metastases and non-MCPyV etiopathogenesis, and emerging markers in need of further exploration.
Merkel cell polyomavirus (MCPyV)
As noted above, MCPyV infection and clonal integration plays a causative role in MCC tumor development. The prevalence of MCPyV has shown geographical variability with some centers demonstrating positivity in up to 80% of MCCs and others documenting much lower rates of positivity, reflecting the greater tendency for solar UV radiation exposure with proximity to the equator (e.g., Western Australia) (9). Seropositivity for MCPyV has been correlated with improved prognosis in some studies but has not consistently been shown to correlate with outcome in other investigations (8). Nonetheless, MCPyV blood antibody titers have been shown to correlate with tumor burden and may be a useful clinical surveillance tool (3). Positive immunohistochemical staining for the MCPyV encoded LT-ag in MCC is strongly associated with MCPyV positivity and helps exclude metastatic neuroendocrine carcinoma, however, negative staining does not exclude the possibility of MCPyV+ MCC (3). In a study that evaluated two distinct MCPyV LT-ag antibodies by immunohistochemistry (CM2B4 and Ab3) and quantitative PCR for MCPyV DNA using the LT4 primer set, staining for CM2B4 showed the highest overall sensitivity (0.882) and specificity (0.943) for MCPyV (10). Of the 282 MCC’s tested, 19% were negative for MCPyV and were clinically more aggressive with significantly increased risk of disease progression and death (hazard ratios 1.77 and 1.85, respectively) in multivariate analyses that included age, sex, and immune status (10).
Immune status
Consistent with a viral etiopathogenesis in many cases, immunodeficiency is a risk factor for MCC development. However, baseline host immune status is also predictive of survival, with immunosuppression correlating with poorer MCC-specific patient survival (2). Immunocompetence and the presence of brisk tumor-infiltrating lymphocytes (particularly at the tumor periphery), typically of the CD3+CD8+ T-cell cytotoxic immune phenotype, correlate with an improved overall rate of survival (8,11). Prolonged survival has also been demonstrated for primary MCCs with increased CD8+ cytotoxic T-cell gene expression (2). CD8+ T-cell mediated cellular immunity may also underlie spontaneous regression of primary and/or metastatic MCC and associate with improved prognosis (3).
Primary tumor parameters
Primary tumor parameters that are typically included in MCC diagnostic reports but not formally incorporated among eighth AJCC staging criteria are the following: tumor thickness, mitotic rate, growth pattern and lymphovascular invasion (LVI). As with other solid tumors of the skin, such as Breslow thickness in melanoma, a greater measured tumor thickness in MCC portends a significantly poorer disease-free survival (8). A mitotic index of >10 per high power field correlates with poor prognosis. Similarly, an infiltrative tumor growth pattern, as opposed to circumscribed nodular growth in the dermis, correlates with poor survival. The presence of both peritumoral and intratumoral LVI (Figure 1C) in MCC associates with a higher frequency of nodal metastasis (3,8). LVI has not been formally included among AJCC staging parameters in part due to the inconsistent reporting of this finding as well as nonuniform utilization of IHC which may alter the rate of detection (12). However, the presence of LVI has been correlated with SLN metastasis and independently associated with worse recurrence-free and disease-specific survival (13). In addition, presence of isolated MCC cells close to tissue margins and distinct from the main tumor mass may underlie the tendency for locally recurrent disease, often occurring within 2 years of initial MCC diagnosis (3).
KIT (CD117)
Tumor cell expression of cKIT (CD117) in MCC has been demonstrated in 53–95% of MCC’s and has been shown to correlate with poor survival (2,14). In one study, 83.3% of 30 MCC’s showed KIT protein expression (high intensity of immunohistochemical staining). Compared to those with low intensity staining, there was a trend towards decreased overall survival at 2 and 5 years (15). However, these data did not reach statistical significance (15). Other groups have demonstrated that strong KIT expression correlates with a higher mitotic rate and LVI, with higher rates of KIT protein expression and KIT exon 11 somatic gene mutations detected among MCC patients that died of disease (14). A potentially confounding factor is that KIT positivity was shown to correlate with p53 positivity and MCPyV- MCC which may, in part, account for the observed prognostic differences (16). Further studies to investigate the role of KIT protein expression in MCC progression and/or metastasis are needed.
Ki-67
The role of the Ki-67 proliferation index in estimating survival among MCC patients has been variably described, in part due to inconsistent methods and lack of established cutoffs (17). High Ki-67 proliferation rates have been associated with poor disease-specific survival, particularly between stage I/II and stage III/IV patient groups, but these findings did not reach statistical significance as an independent factor in multivariate analyses that included age and sex (17).
p63
Tumor expression of p63 in MCC, present in roughly one-third of cases, was observed to predict risk of death in a number of early investigations, regardless of MCPyV status (3,18). However, more recent multivariate analyses have not reported as high a rate of p63 expression among MCCs and have failed to confirm this marker as an independent variable predictive of poor outcome when tumor stage at presentation is accounted for (2,9,12). Additional studies are needed to define p63 as predictive of metastasis or survival.
Tumor site
Special prognostic considerations related to tumor site for MCC include the ease of achieving a complete excision with negative surgical margins, and other anatomical factors, such as structural features or clinically inconspicuous sites challenging early detection. MCCs commonly arise at sun-exposed sites of the head and neck. On the scalp, large MCCs correlate with likelihood of distant metastases. On the ear, MCC shows the highest rate of metastasis to lymph nodes. On the lip, MCC often extends deep to the subcutis with involvement of underlying muscle, cartilage and bone, and negatively impacts prognosis. Vulvar or perianal sites of involvement portend the poorest prognosis (8).
Imaging
Given the tendency for nodal involvement, imaging studies such as ultrasonography and PET-CT are an essential clinical staging tool useful for predicting outcome and informing MCC patient management (3). Emerging imaging modalities such as single-photon CT imaging, use of intraoperative gamma camera technology, and somatostatin analog-based imaging methods may enhance the clinical detection of involved lymph nodes and better characterize the localization/anatomical extent of MCC (12). The importance of optimizing the capabilities of imaging is underlined by the key role that lymph node status and metastasis play among staging parameters that determine patient prognosis (15).
KC
Background
KC represents the overwhelming majority of NMSC. KC includes BCC and cutaneous squamous cell carcinoma (cSCC). KC is the most frequently diagnosed cancer in the United States (US) and in fair-skinned populations. BCC is the most common human cancer, representing up to 75–80% of KC, although the ratio of BCC to cSCC is decreasing as the population ages (19,20). With over 5 million KC diagnoses yearly in the US and an increasing incidence, KC represents a large public health burden (20). KC most commonly occurs on the head and neck, where significant morbidity arises from destructive surgical procedures that may involve removal of the eye, nose or facial bones. cSCC has a non-negligible rate of metastatic disease (<3% to 7%), and given its high incidence, describing biomarkers that predict which tumors may metastasize is important to prevent death. BCC is often locally destructive, but it metastasizes only in very rare cases (21).
BCC is a basaloid tumor thought to arise from a pluripotent precursor cell associated with follicular units and displays a variety of growth patterns. The histological differential diagnosis includes other basaloid tumors with follicular, eccrine or sebaceous differentiation, particularly trichoepithelioma (22). cSCC histologically ranges from a glassy eosinophilic tumor with a low nuclear to cytoplasmic ratio, large amounts of cystic keratin and an endophytic, pushing invasion pattern to a highly infiltrative, poorly differentiated carcinoma set in a desmoplastic stroma (23).
Etiopathogenesis
Although BCC and cSCC are typically histopathologically distinct, both are largely driven by ultraviolent (UV) radiation (particularly UV-A), which leads to the high mutational burden seen in KC. Other risk factors for KC include immunosuppression, skin type, ionizing radiation, environmental exposures such as arsenic (BCC), and age. BCC occurs at a younger age than SCC, the latter being more closely associated with chronic cumulative sun exposure (24).
Approximately 70% of sporadic BCCs have demonstrable mutations in the PTCH gene on chromosome 9q22, a gene with tumor suppressor activity in the highly conserved Hedgehog (Hh) signaling pathway of organogenesis. Likewise, germline mutations in PTCH result in the nevoid BCC (Gorlin) syndrome (25). UV-driven mutations in other genes that interact with the Hh pathway contribute to the pathogenesis of the remaining subset of BCCs. For example, alterations in the oncogene SMO upregulates the Hh pathway (26). Other polymorphisms associated with DNA repair or mutagen detoxification have also been associated with increased risk of BCC.
Immunosuppression plays a significant role in the development of KC, particularly aggressive cSCC (23). Multiple forms of immunosuppression (primary and acquired immunodeficiency syndromes, autoimmune disease, hematologic malignancy) can increase the risk of KC, but solid organ transplantation in particular may bear up to a 200 times risk of developing KC (19).
Germline mutations in other genes associated with DNA repair and chromosomal stability also increase the risk of the development of all KC. These include xeroderma pigmentosum, Rothmund-Thompson and Bloom syndromes (24).
Staging of KC
Staging of KC essentially refers to the staging of cSCC, as BCC is rarely staged due to its very low propensity for metastasis and nearly non-existent disease specific death rate. Validation of staging systems has been generally difficult since national cancer registries have historically not included KC (27).
Two major staging systems are used to stage cSCC in the US: the American Joint Committee on Cancer (AJCC) system and the Brigham and Women’s Hospital (BWH) method. The AJCC published its eighth edition in 2017, listing cSCC as a head and neck tumor; other special anatomic sites use separate staging classifications (27).
Most cSCC is local disease and tumor size still forms the backbone of the AJCC staging system. Tumor diameter of <2 cm defines the T1 category, and tumors with a dimension of greater than or equal to 2 cm but less than 4 cm are considered T2. T3 tumors are 4 cm or larger or tumors of any size with one high risk feature [invasion >6 mm or into subcutis, minor bone erosion, perineural invasion (PNI)]. In this staging system, PNI must be deeper than the dermis, involve a nerve 0.1 mm in diameter or greater, or be clinically or radiologically evident. T4a tumors show gross cortical bone or marrow invasion and T4b tumors invade the skull base or involve skull base foramen (28). This staging system has struggled to distinguish between the T2 and T3 categories in terms of disease related events, leading investigators to attempt to further sub-stratify medium sized tumors regarding the risk for poor outcome.
The BWH staging system is based on number of high-risk features seen in a given tumor (0= T1, 1= T2a, 2–3= T2b, all 4 or bone invasion = T3) (29). The BWH high-risk features are defined as the following: poorly differentiated histology, tumor diameter of 2 cm or greater, PNI, and deep invasion (beyond subcutis but excluding bone). This system allows for better risk stratification of which AJCC T2 tumors may locally recur or metastasize. Nodal (N) and metastatic (M) staging is not included in this system due to the rarity of these events.
Both systems, and others that have been proposed [Breuninger and colleagues, the International Union Against Cancer (Union for International Cancer Control, UICC)], are under continued evaluation and refinement since >95% of cSCC does not metastasize (27). Multiple nodal staging systems have been described, since the AJCC system showed little difference in overall survival between adjacent N stages. Interestingly, most cases of metastatic cSCC show extra-nodal extension.
Biomarkers
KC show a UV-driven high mutational burden, with cSCC having the highest mutation rate per megabase pair of any cancer (30). However, certain genetic biomarkers including mutations in specific tumor suppressor genes, have been associated with more aggressive disease. These are better described in cSCC given the higher risk for metastatic disease. Anatomic parameters such as differentiation, size and site have also been established as important predictive and prognostic factors in cSCC and BCC.
Primary tumor parameters
Certain histologic subtypes of cSCC (desmoplastic, Figure 1D) portend a much higher risk (up to 10 times) of metastatic disease compared to others (23). Histologic differentiation may be difficult to study due to the subjectivity in grading, but poor differentiation has consistently been associated with poor prognosis including triple the recurrence rate and double the metastatic risk in cSCC (31). Extension beyond the subcutis (or tumors 6 mm or greater in depth) is associated with a >25% risk of both local recurrence and metastases (23,29). Tumors larger than 2 cm have twice the risk of recurrence and three times the risk of metastasis when compared to tumors under 2 cm. Particular anatomic sites (ear, lip) and background diseases (cSCC arising in a scar) have 10–26% risk of metastasis compared to the overall rate of 1.5–5% (23). BCC with an infiltrative, morpheaform or desmoplastic histologic appearance/growth pattern also behaves more aggressively and is more likely to locally recur (24).
Perineural invasion
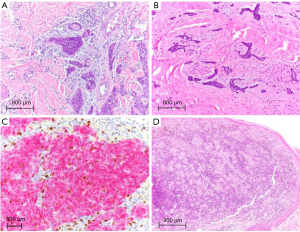
PNI (Figure 2A) has long been associated with a markedly increased risk of metastatic cSCC (23,27). Recently, a study of over 1000 tumors showed lymph node metastasis in 3% of non-desmoplastic cSCCs without PNI and 29% in desmoplastic SCCs with PNI. Local recurrence increased from 3% to 64% and overall tumor specific death was 54% in desmoplastic tumors with perineural invasion, making identification of PNI critical in the prognostication of cSCC and the decision for adjuvant treatment (32). In particular, large caliber nerve invasion (0.1 mm or larger) is associated with nodal metastasis and mortality. BCC with PNI (Figure 2B) is locally aggressive, particularly where it more commonly occurs on the head and neck, but PNI does not have the same well-established increased metastatic or mortality risks as cSCC with PNI (21,33).
Human papillomavirus (HPV)
Unlike the clearly defined role of high-risk HPV in oropharyngeal and anogenital SCC, the association of HPV and cSCC is much less clear. ßHPV subtypes have been detected in a widely variable number of cSCC and evidence of HPV is more commonly found in tumors arising in immunosuppressed patients (19). However, these HPV types (types 8, 9, 15) have not been shown to be transcriptionally active in cSCC (23). Until recently, the precise role they play in the induction of such tumors was not known. Studies have now demonstrated that the loss of T cell immunity against commensal papillomaviruses in immunosuppressed patients (as opposed to a direct oncogenic effect of HPV) likely underlies the increased risk of skin cancer (34). Further investigations are needed to elucidate the role of HPV in cSCC.
TP53
Mutations in TP53 appear to be an early event in the development of cSCC and have been found in precursor lesions such as actinic keratosis, further supportive of this hypothesis. TP53 is the most commonly mutated tumor suppressor gene in cSCC (up to 90% of cases) (23,35). In BCC, TP53 mutations are less commonly encountered (~50%) and appear to be a later event that some authors postulate is crucial in tumor progression (25).
NOTCH, CDKN2A, TERT
Alterations in genes affecting cell signaling and proliferation including Neurogenic Locus Notch Homolog Protein (NOTCH) 1 and 2 and cell cycle inhibitors like CDKN2A and are also common in cSCC (35,36). Small studies have shown higher rates of these mutations in metastatic cSCC than in high-risk, non-metastatic cSCC, but some mutations (such as those in NOTCH 1) are reported at such a high rate of cSCC (~80%) it is unclear if they truly drive metastatic potential. Similar mutations have also been found in adjacent uninvolved skin, making true prognostic significance difficult to ascertain (35).
Telomerase reverse transcriptase gene (TERT) promoter mutations, which are common in cutaneous melanomas, are also found in a significant proportion (approximately a third) of cSCC, including those with only in situ disease (37). Despite the high prevalence overall, TERT promotor mutations in a recent study were independently associated with a higher rate of local recurrence and nodal disease, although the statistical significance was uncertain due to the low number of metastatic cases, particularly those without TERT promoter mutations.
Melanoma
Background
Melanoma is a malignant skin tumor with a rising incidence and high mortality rate worldwide despite screening campaigns and public health awareness (38). Early diagnosis and treatment are crucial for optimal patient outcome. The understanding of prognostic and predictive biomarkers is rapidly evolving and has the potential to further improve melanoma patient survival through targeted therapeutics.
Etiopathogenesis
Melanomagenesis occurs through a number of concurrent pathways and is closely linked to exposure to ultraviolet radiation, particularly in fair-skinned individuals, and often evolves from precursor nevi (39). Key molecular underpinnings in melanoma development include mutations in NRAS, BRAF(V600E), TERT, GNAQ, GNA11, BAP1, and CDKN2A among others (39). The acquisition of greater numbers of genetic mutations generally correlates with disease progression from benign nevus to malignant melanoma (39). However, the progression is nonlinear and patient risk stratification remains somewhat unpredictable owing in part to the broad range of melanoma tumor subtypes (e.g., superficial spreading, nodular, acral lentiginous, and lentigo maligna melanoma) and difficult-to-classify melanocytic tumors of uncertain malignant potential (MELTUMP). The WHO classification of skin tumors incorporates epidemiological, clinical, pathological, and genomic features into the classification of melanoma (39).
AJCC staging
The 5-year survival rate for localized melanoma (AJCC stage I) is 98.5%, which drops to 62.5% in patients with regional metastasis and to 19.9% in patients with distant metastases (AJCC stage IV) (40). In addition to patient age, gender, and tumor localization, important melanoma pathological prognostic factors include Breslow thickness, mitotic index and ulceration (41). The primary tumor thickness (T category) cutoff of >0.75 mm for melanoma originally proposed by Alexander Breslow in 1970 was later updated to 1.0 mm and has now reverted to 0.8 mm in the latest AJCC staging (39). Higher mitotic rates correlate with decreased survival and predict a higher risk of metastasis particularly in thin melanomas (39). Of note, despite the importance of mitotic index as a prognostic marker, it was not included among AJCC staging parameters in the latest edition due to conflicting data, although many pathologists continue to mention mitotic rate (Figure 2C) in diagnostic reports of primary melanoma (39,42,43). In contrast, the presence of and particularly the measured width of ulceration continues to be recognized as a marker of poor prognosis (higher nodal positivity rate) in the latest AJCC staging system (39,42). The presence of brisk tumor-infiltrating lymphocytes (TILs, Figure 2D) reflecting host immune response portends favorable prognosis in melanoma (39). Furthermore, host immune responses have been leveraged for the development of immune checkpoint inhibitors, including anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and anti-programmed cell death 1 (PD-1) therapies, in advanced melanoma (39). Sentinel lymph node (SLN) status remains a key melanoma prognostic factor for survival and guiding adjuvant systemic therapy (39). In addition, SLN tumor burden is predictive of metastasis to non-SLNs within the regional node field (43).
Biomarkers
Prognostic and predictive biomarkers ideally aid in detection of early disease and/or in prediction of biological behavior (propensity to recur or metastasize) and tumor responsiveness (and/or resistance) to therapy (38). In practice, melanoma tumor characteristics incorporated into the AJCC staging system do not entirely align with patient outcome imparting a degree uncertainty in assessing the biological potential of this complex tumor type. Molecular profiling of primary melanomas, such as with the 31-gene expression profile test, can augment prognostic accuracy (44).
Lactate dehydrogenase (LDH)
LDH is an enzyme responsible for catalyzing the conversion of pyruvate to lactate under anaerobic conditions (38). In melanoma, elevated serum LDH levels correlate with worse overall survival (38). Despite its non-specificity, the use of LDH as a melanoma serum biomarker is strongly supported by data and represents the only prognostic serum biomarker acknowledged by the AJCC. In the eighth edition cancer staging manual, the M1 category of distant metastases is subdivided by serum LDH level (40). LDH is also well-suited as a predictive biomarker with high levels correlated with improved outcome following ipilimumab treatment of advanced melanoma (38). In addition, serum LDH levels are reflective of disease burden, with regression correlating with decreased levels, and progression correlating with an increase over the baseline serum level of LDH, favoring the use of this marker for disease monitoring. Care must be taken in the interpretation of LDH serum levels as laboratory values may be falsely elevated in the setting of non-malignant disorders that result in cell death or damage, such as hemolysis, rhabdomyolysis, and myocardial infarction (38).
S100
Members of the S100 family of calcium-binding proteins, S100B, S100A4, and S100A9, associate with melanoma progression and may serve as therapeutic targets (38). High circulating S100B levels have been shown to correlate with Breslow thickness and tumor burden (38). In European studies, S100B is proposed as a biomarker of disease progression that rivals LDH (38). Therapeutic blockade of S100A4 in melanoma xenograft models has been shown to inhibit tumor growth and angiogenesis (38). S100A9, when dimerized with S100A8, plays a role in melanoma cell proliferation and migration and high levels are predictive of therapeutic resistance to ipilimumab (38). Thus, S100 is a useful serum biomarker of tumor progression, relapse or metastasis, particularly in patients with advanced melanoma (41). However, as a tissue marker, S100 immunohistochemical staining is unable to differentiate benign melanocytic proliferations from malignant melanoma and its expression does not inform prognosis (41).
BRAF
In melanocytes, activating mutations in BRAF (v-raf murine sarcoma viral oncogene homolog B1), a downstream target of c-KIT, stimulate MITF (microphthalmia-associated transcription factor) gene expression and activity via MAPK pathway activation (MEK/ERK phosphorylation) (45). At the protein level, MITF and c-KIT demonstrate significantly reduced expression in metastatic melanoma as compared to benign nevi and primary melanoma, respectively (44). BRAF gene mutation is a key driver of the transformation of melanocytes to melanoma and is present in 35–60% of primary cutaneous and conjunctival melanomas, in contrast to uveal melanoma and other mucosal melanoma subtypes which demonstrate other key driver mutations (46,47). Melanomas harboring mutant BRAF demonstrate poorer patient prognosis and overall survival (47). The vast majority of BRAF mutations involve glutamic acid (E) replacement of valine (V) at amino acid position 600 (V600E). Mutant BRAFV600E targeting with combined BRAF/MEK inhibition significantly improves progression-free survival and overall survival in patients with metastatic disease (46,47). Thus, BRAF is both a strong predictive biomarker and therapeutic target in patients with advanced melanoma (47).
NRAS, KIT
NRAS and KIT are listed among College of American Pathology (CAP) approved melanoma biomarkers (48). Roughly 20% of melanomas harbor NRAS mutations, with the majority occurring in exon 3, codons 60 and 61, and some occurring in exon 2, codons 12 and 13 (48). Of note, NRAS and BRAFV600E mutations in melanoma are typically mutually exclusive (48) and clinical investigations of NRAS as a predictive biomarker in melanoma are ongoing. KIT mutations are identified in <5% of melanomas, including those arising in mucosal, acral and chronic actinically-damaged skin (48). In clinical trials, targeted KIT inhibitors have shown promise, with response documented in tumors with alterations in the L576 and K642 hotspots (48).
Tyrosinase
Tyrosinase is widely regarded as a melanoma enzyme biomarker responsible for catalyzing the conversion of L-tyrosine to L-DOPA and L-DOPA to dopaquinone in melanin biosynthesis. In melanoma, high tyrosinase levels correlate with presence of circulating tumor cells and likelihood of metastasis (38). However, the utility of RT-PCR detection of tyrosinase mRNA as a biomarker is clinically limited due to methodological differences between studies resulting in a broad range of cut-off values (38).
Cyclooxygenase-2 (Cox2)
Overexpression of Cox2 in melanoma has been reported to be a marker of poor prognosis that correlates with tumor thickness, ulceration and nodal metastasis (38). In addition, Cox2 may serve as a predictive marker of treatment resistance, such as to chemotherapy and radiotherapy (38).
Matrix metalloproteinases (MMP)
MMPs encompass a family of proteins, such as collagenases and stromelysins, involved in an array of biological processes ranging from wound healing and chronic inflammation to neoplasia (38). MMP-2 and MMP-9, among others, are known to play a role in melanoma disease progression, associate with poor survival, and in some studies, correlate with nodal metastasis (38).
B-cells
While the role of T-cells in the tumor infiltrating lymphocyte (TIL) response in melanoma is well established (see AJCC Staging above), the prognostic role of B-cells has more recently been explored. B-cells comprise approximately 30% of melanoma TILs and their experimental depletion using anti-CD20 immunotherapy was shown to not only reduce the overall TIL response but decreased CD8+ T-cell numbers (49). In some studies, melanoma associated B-cells were predictive of response to anti-PD-1 therapy and longer overall survival (49,50). In contrast, plasma cells, when present in sheets/clusters, were associated with worse prognosis with some reports showing that plasma cell-rich primary cutaneous melanomas were significantly thicker, more mitotically active, ulcerated, and occurred in older aged patients (51). Further studies are needed to more fully characterize the multifaceted role of humoral immunity in melanoma prognosis and immunotherapeutic response prediction (50).
Conclusions
In summary, we review recent advances in the literature on established and emerging predictive and prognostic biomarkers in MCC, melanoma, and KCs, including BCC and squamous cell carcinoma. Awareness of key predictive and prognostic biomarkers augments traditional pathologic staging parameters for improved precision in therapeutic management and accurate prediction of response in patients with malignancies of the skin.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors [Mari Mino-Kenudson and Yin (Rex) P. Hung] for the series “Predictive and Prognostic Biomarkers in Tumors” published in Precision Cancer Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review Reporting Checklist. Available at: http://dx.doi.org/10.21037/pcm-21-6
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/pcm-21-6). The series “Predictive and Prognostic Biomarkers in Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hughes MP, Hardee ME, Cornelius LA, et al. Merkel cell carcinoma: Epidemiology, target, and therapy. Curr Derm Rep 2014;3:46-53. [Crossref] [PubMed]
- Tetzlaff MT, Harms PW. Danger is only skin deep: aggressive epidermal carcinomas. An overview of the diagnosis, demographics, molecular-genetics, staging, prognostic biomarkers, and therapeutic advances in Merkel cell carcinoma. Mod Pathol 2020;33:42-55. [Crossref] [PubMed]
- Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers 2017;3:17077. [Crossref] [PubMed]
- Feng H, Shuda M, Chang Y, et al. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008;319:1096-100. [Crossref] [PubMed]
- Tai P, Park SY, Nghiem PT. Staging and treatment of Merkel cell carcinoma. In: UpToDate, Waltham, MA. Accessed on July 20, 2020.
- Amin MB, Edge SB, Greene FL. AJCC cancer staging manual, 8th edition. Merkel cell carcinoma, chapter 46. Switzerland: Springer, 2017:549-61.
- Harms, KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol 2016;23:3564-71.
- Trinidad CM, Torres-Cabala CA, Prieto VG, et al. Update on eighth edition American Joint Committee on Cancer classification for Merkel cell carcinoma and histopathological parameters that determine prognosis. J Clin Pathol 2019;72:337-40.
- Dabner M, McClure RJ, Harvey NT, et al. Merkel cell polyomavirus and p63 status in Merkel cell carcinoma by immunohistochemistry: Merkel cell polyomavirus positivity is inversely correlated with sun damage, but neither is correlated with outcome. Pathology 2014;46:205-10. [Crossref] [PubMed]
- Moshiri AS, Doumani R, Yelistratova L, et al. Polyomavirus-negative Merkel cell carcinoma: A more aggressive subtype based on analysis of 282 cases using multimodal tumor virus detection. J Invest Dermatol 2017;137:819-27. [Crossref] [PubMed]
- Feldmeyer L, Hudgens CW, Ray-Lyons G, et al. Density, distribution, and composition of immune infiltrates correlate with survival in Merkel cell carcinoma. Clin Cancer Res 2016;22:5553-63. [Crossref] [PubMed]
- Bichakjian CK, Nghiem P, Johnson T, et al. 46. Merkel cell carcinoma. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th edition. 2016.
- Harounian JA, Molin N, Galloway TJ, et al. Effect of sentinel lymph node biopsy and LVI on Merkel cell carcinoma prognosis and treatment. Laryngoscope 2021;131:E828-35. [Crossref] [PubMed]
- Swick BL, Srikantha R, Messingham KN. Specific analysis of KIT and PDGFR-alpha expression and mutational status in Merkel cell carcinoma. J Cutan Pathol 2013;40:623-30. [Crossref] [PubMed]
- Andea AA, Patel R, Ponnazhagan S, et al. Merkel cell carcinoma: correlation of KIT expression with survival and evaluation of KIT gene mutational status. Hum Pathol 2010;41:1405-12. [Crossref] [PubMed]
- Husein-ElAhmed H, Ramos-Pleguezuelos F, Ruiz-Molina I, et al. Histological features, p53, c-Kit, and Poliomavirus status and impact on survival in Merkel cell carcinoma patients. Am J Dermatopathol 2016;38:571-9. [Crossref] [PubMed]
- La Rosa S, Bonzini M, Sciarra A, et al. Exploring the prognostic role of Ki67 proliferative index in Merkel cell carcinoma of the skin: Clinico-pathologic analysis of 84 cases and review of the literature. Endocr Pathol 2020;31:392-400. [Crossref] [PubMed]
- Hall BJ, Pincus LB, Yu SS, et al. Immunohistochemical prognostication of Merkel cell carcinoma: p63 expression but not polyomavirus status correlates with outcome. J Cutan Pathol 2012;39:911-7. [Crossref] [PubMed]
- Nagarajan P, Asgari MM, Green AC, et al. Keratinocyte carcinomas: current concepts and future research priorities. Clin Cancer Res 2019;25:2379-91. [Crossref] [PubMed]
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081-6. [Crossref] [PubMed]
- Laga AC, Schaefer IM, Sholl LM, et al. Metastatic Basal Cell Carcinoma. Am J Clin Pathol 2019;152:706-17. [Crossref] [PubMed]
- McDaniel B, Badri T, Steele RB. Basal Cell Carcinoma. 2020 Nov 20. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol 2018;78:237-47. [Crossref] [PubMed]
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol 2019;80:303-17. [Crossref] [PubMed]
- Madan V, Hoban P, Strange RC, et al. Genetics and risk factors for basal cell carcinoma. Br J Dermatol 2006;154:5-7. [Crossref] [PubMed]
- de Zwaan SE, Haass NK. Genetics of basal cell carcinoma. Australas J Dermatol 2010;51:81-92. [Crossref] [PubMed]
- Bander TS, Nehal KS, Lee EH. Cutaneous squamous cell carcinoma: updates in staging and management. Dermatol Clin 2019;37:241-51. [Crossref] [PubMed]
- Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:122-37.
- Karia PS, Jambusaria-Pahlajani A, Harrington DP, et al. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women's Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol 2014;32:327-34. [Crossref] [PubMed]
- Mulvaney PM, Schmults CD. Molecular prediction of metastasis in cutaneous squamous cell carcinoma. Curr Opin Oncol 2020;32:129-36. [Crossref] [PubMed]
- Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol 2008;9:713-20. [Crossref] [PubMed]
- Haug K, Breuninger H, Metzler G, et al. Prognostic impact of perineural invasion in cutaneous squamous cell carcinoma: results of a prospective study of 1,399 tumors. J Invest Dermatol 2020;140:1968-75. [Crossref] [PubMed]
- Balamucki CJ, DeJesus R, Galloway TJ, et al. Impact of radiographic findings on for prognosis skin cancer with perineural invasion. Am J Clin Oncol 2015;38:248-51. [Crossref] [PubMed]
- Strickley JD, Messerschmidt JL, Awad ME, et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019;575:519-22. [Crossref] [PubMed]
- Campos MA, Lopes JM, Soares P. The genetics of cutaneous squamous cell carcinogenesis. Eur J Dermatol 2018;28:597-605. [PubMed]
- Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res 2014;20:6582-92. [Crossref] [PubMed]
- Campos MA, Macedo S, Fernandes M, et al. TERT promoter mutations are associated with poor prognosis in cutaneous squamous cell carcinoma. J Am Acad Dermatol 2019;80:660-9.e6. [Crossref] [PubMed]
- Belter B, Haase-Kohn C, Pietzsch J. Biomarkers in malignant melanoma: recent trends and critical perspective. In: Ward WH, Farma JD. editors. Cutaneous melanoma: etiology and therapy. Brisbane (AU): Codon Publications, 2017. Dec 21.
- Rawson RV, Scolyer RA. From Breslow to BRAF and immunotherapy: evolving concepts in melanoma pathogenesis and disease progression and their implications for changing management over the last 50 years. Hum Pathol 2020;95:149-60. [Crossref] [PubMed]
- Trinidad CM, Torres-Cabala CA, Curry JL, et al. Update on eighth edition American Joint Committee on Cancer classification for cutaneous melanoma and overview of potential pitfalls in histological examination of staging parameters. J Clin Pathol 2019;72:265-70.
- Utikal J, Schadendorf D, Ugurel S. Serologic and immunohistochemical prognostic biomarkers of cutaneous malignancies. Arch Dermatol Res 2007;298:469-77. [Crossref] [PubMed]
- Chopra A, Sharma R, Rao UMN. Pathology of melanoma. Surg Clin N Amer 2020;100:43-59. [Crossref] [PubMed]
- Scolyer RA, Rawson RV, Gershenwald JE, et al. Melanoma pathology reporting and staging. Mod Pathol 2020;33:15-24. [Crossref] [PubMed]
- Greenhaw BN, Covington KR, Kurley SJ, et al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J Am Acad Dermatol 2020;83:745-53. [Crossref] [PubMed]
- Nazarian RM, Prieto VG, Elder DE, et al. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol 2010;37:41-7. [Crossref] [PubMed]
- Bol KF, Donia M, Heegaard S, et al. Genetic biomarkers in melanoma of the ocular region: what the medical oncologist should know. Int J Mol Sci 2020;21:5231-45. [Crossref] [PubMed]
- Ny L, Hernberg M, Nyakas M, et al. BRAF mutational status as a prognostic marker for survival in malignant melanoma: a systematic review and meta-analysis. Acta Oncol 2020;59:833-44. [Crossref] [PubMed]
- CAP melanoma biomarker reporting template, Melanoma Biomarkers 1.0.0.2, Available online: https://www.cap.org/protocols-and-guidelines/cancer-reporting-tools/cancer-protocol-templates (Accessed January 24, 2021).
- Griss J, Bauer W, Wagner C, et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat Commun 2019;10:4186. [Crossref] [PubMed]
- Chiaruttini G, Mele S, Opzoomer J, et al. B cells and the humoral response in melanoma: The overlooked players of the tumor microenvironment. Oncoimmunology 2017;6:e1294296. [Crossref] [PubMed]
- Bosisio FM, Wilmott JS, Volders N, et al. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod Pathol 2016;29:347-58. [Crossref] [PubMed]
Cite this article as: Stagner AM, Nazarian RM. A narrative review of predictive and prognostic biomarkers in skin tumors. Precis Cancer Med 2021;4:22.

