
The role of immunotherapy in metastatic triple negative breast cancer: a narrative review of the current clinical trials
Introduction
Triple negative breast cancer (TNBC) is an aggressive subtype of breast cancer, in which the tumor cells do not express estrogen (ER) or progesterone (PR) receptors, and do not overexpress the human epithelial growth factor 2 (HER2) receptor. TNBC occurs in around 15–20% of breast cancer cases, is more likely to present at an advanced stage, has higher relapse rates, and has a worse prognosis than other breast cancer types (1-3). Unlike patients with hormone or HER2 positive breast cancers, there are no targeted therapies available for the majority of patients with TNBC (4). On average, patients with metastatic TNBC (mTNBC) have an overall survival (OS) of 12–16 months with treatment, and the mainstay of treatment for most patients remains chemotherapy (4-6). Based on the aggressive nature of this disease and challenges in treatment for these patients, there has been a focus on identifying novel and more effective combination therapies to improve long-term outcomes.
Recently, numerous studies have focused on how malignancies evade the immune system. The programmed cell death 1 (PD-1) receptor pathway has been found to mediate the immune response in several malignancies including melanoma, renal cell carcinoma, and lung cancer, among others (7-9). As depicted in Figure 1, PD-1 is a surface membrane receptor expressed by several immune cells, including T cells. PD-1 binds to programmed-death ligand 1 and 2 (PD-L1 and PD-L2), which decreases T cell function and survival, terminating the immune response (10). Either the tumor itself or cells in the tumor micro-environment can express PD-L1, both of which can prevent an immune response to the malignancy.
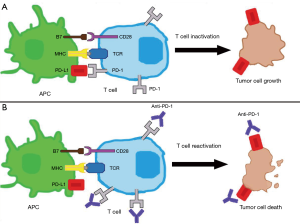
There are several factors that would make TNBC a good candidate for immune-based therapies. TNBC has the highest mutational burden of any breast cancer subtype (11); which in theory, allows for the potential of increased neo-antigens to be targeted by the immune system. TNBC is also associated with higher levels of stromal tumor infiltrating lymphocytes (TILs), which has proven to be an important prognostic factor for early TNBC (12). The higher levels of TILs suggest an innate immune response to TNBC. A meta-analysis of six randomized trials showed that for every 10% increase in stromal TILs, there was a 17% relative risk reduction in death, and that TILs are associated with OS in multi-variate analysis for patients with early TNBC (12). In TNBC, the presence of PD-1 on TILs is much higher than on the tumor cells itself, and appears to be more important for prognostic outcomes (13,14). This suggests that immunotherapy may have a prominent role in TNBC. There has been a focus of investigating the role of immunotherapy in mTNBC. In this review, we examine the recent trials and role of single agent immunotherapy and combination immunotherapy with chemotherapy, radiation therapy and novel therapies. We also discuss the current standard of care for first-line mTNBC patients, the potential role for biomarkers, and future direction for advancing care for patients with mTNBC. We present the following article in accordance with the narrative review checklist (available at: http://dx.doi.org/10.21037/pcm-20-58).
Methods
The database PubMed was searched for publications between January 1, 2000 and October 1, 2020 to identify clinical trials. The search strategy used was ((triple negative breast cancer OR TNBC) AND (immunotherapy OR PD-1 OR PD-L1 OR CTLA-4)). Published studies were included if they had more than 25 patients with mTNBC in phase 1 studies, or 15 patients for phase 2 and 3 trials. Relevant trials presented as conferences but not published, were identified by searching clinicaltrials.gov. The condition searched was breast cancer, and individual immunotherapy drug names were used in other terms to identify relevant clinical trials. Clinical trials were then individually searched to see if results were presented at conferences. Immunotherapy drugs searched were: atezolizumab, avelumab, camrelizumab, cemiplimab, dostarlimab, durvalumab, ipilimumab, nivolumab, pembrolizumab, sintilimab, tislelizumab and toripalimab. Trials were excluded if insufficient data was provided to identify the line of therapy in which immunotherapy was given, or immunotherapy was not the focus of the paper.
Phase 1 data
The flagship trial to evaluate immunotherapy in mTNBC was the phase I KEYNOTE-012 study (ClinicalTrials.gov identifier NCT01848834) (15). This multi-cohort study included gastric, urothelial, TNBC and head and neck cancers. For the mTNBC patients, eligibility required PD-L1 positivity defined as ≥1% expression in the tumor cells or PD-L1 expression in the stoma. Of the 111 patients screened, 32 enrolled in the study. Patients were heavily pre-treated, with the median number of prior systemic treatment lines being two. Patients were treated with the humanized monoclonal antibody (mAB) against PD-1, pembrolizumab. The overall response rate (ORR) was 18.5%, with 1 (3.7%) complete response (CR), 4 (14.8%) partial responses (PRs), and 7 (25.9%) patients had stable disease (SD). The median progression free survival (mPFS) was 1.9 months, and the median OS (mOS) was 11.2 months.
Atezolizumab, a fully humanized mAB against PD-L1, was evaluated in a phase I trial in patients with mTNBC (NCT01375842) (16). Patients were included regardless of PD-L1 expression; however, patients were measured for PD-L1 positivity. In this trial, PD-L1 positive was defined as ≥1% of TILs. Individuals were evaluated for first-line or later treatment. The ORR for the entire cohort (n=166) was 10%, 24% in those with first-line treatment and 6.4% in second-line or later treatment. No statistical difference in response rates in the first-line or later line settings was detected. Three (2.6%) patients had a CR, and 8 (7.0%) individuals had a PR, and 15 (7.7%) individuals had SD as their best response. Patients with PD-L1 positive disease had better response rates, at 12.1% vs. 0%. The mPFS for the entire cohort was 1.4 months, and was the same in PD-L1 positive patients. PD-L1 positive patients had no statistical difference for OS, (10.1 vs. 6.0 months). Patients with TILs PD-L1 expression of ≥10% had a significantly longer mOS of 12.6 (95% CI: 9.5–15.5) vs. 6.7 (95% CI: 4.9–7.6) months.
In the phase 1b JAVELIN study (NCT01772004) avelumab, another mAB against PD-L1, was evaluated in patients with metastatic breast cancer (MBC) (17). All patients had been treated with a prior taxane and anthracycline chemotherapy, and had less than four lines of prior treatment. Patients were selected regardless of their breast cancer type and PD-L1 status. PD-L1 positive patients were defined as having ≥1% PD-L1 combined positive score (CPS: tumor cells, lymphocytes, and macrophages out of the total number of tumor cells × 100). A total of 168 patients were enrolled, 58 of which were mTNBC patients. The ORR in the TNBC cohort was 5.2% with higher response rates seen in patients with PD-L1 positive patients (22.6% in CPS ≥1% vs. 2.6% in CPS <1%). Only one patient (1.7%) had a CR. The mPFS in the TNBC group was 12.4 months, and the mOS was 9.2 months.
Phase 2 data
Pembrolizumab was further evaluated in the single agent setting in the KEYNOTE-086 trial (NCT02447003). This phase 2 trial evaluated two cohorts of patients with mTNBC, in the first-line (cohort B) and second or later line setting (cohort A) (18,19).
Patients in cohort A were studied in the second or later line setting (18). Patients were included regardless of the PD-L1 status, had at least one line of treatment in the metastatic setting, and had been previously treated with both a taxane and anthracycline at some point in time. Of the 170 patients enrolled, 105 (61.8%) were PD-L1 positive. The ORR in the total population was 5.3%, with 2 (1.2%) patients having a CR and 7 (4.1%) having a PR. Response rates were similar between the PD-L1 positive and negative groups with an ORR of 5.7% and 4.7%, respectively. The progression free survival was 2.0 months in the overall cohort, and 2.0 and 1.9 in the PD-L1 positive and negative groups, respectively. The mOS in the entire cohort was 9.0 months, and 9.7 and 8.8 months in the PD-L1 negative and positive populations.
In cohort B of KEYNOTE-086 trial, 84 patients were enrolled. Patients had no prior treatment in the metastatic setting, and 86.9% received (neo)adjuvant treatment (19). Participating individuals had to have ≥1% PD-L1 CPS. The ORR was 21.4% with 4 patients (4.8%) having a CR, 14 individuals (16.7%) with a PR, and 13 (15.5%) had SD as their best outcome. The median PFS was 2.1 months, and the mOS was 18.0 months.
Phase 3 data
The Keynote 119 trial (NCT02555657) was recently presented in 2019, and is not currently published (20). The study enrolled 622 patients with mTNBC that had been previously treated with anthracycline and taxane chemotherapy, and had 1–2 lines of treatment in the metastatic setting. Patients were randomized 1:1 to either pembrolizumab or physician’s choice of chemotherapy (capecitabine, eribulin, gemcitabine or vinorelbine). Patients were stratified by PD-L1 status using a CPS ≥1%. Disappointingly, the ORR in the entire cohort was 9.6% in the pembrolizumab arm and 10.6% in the chemotherapy arm, which was not statistically different. There was a trend towards a better response rate with increased CPS; however, this was not statistically different from chemotherapy in any of the predefined CPS subgroups. The mPFS was 2.1 months in the entire cohort and for PD-L1 positive patients that received pembrolizumab, and was 3.3 and 3.1 months for patients that received chemotherapy. mOS was not improved with pembrolizumab in the entire cohort when compared to chemotherapy (9.9 vs. 10.8 months). The mOS also did not improve in the CPS ≥1% or CPS ≥10% subgroups. In exploratory analysis, patients with a CPS ≥20% had improved OS with pembrolizumab compared to chemotherapy, 14.9 vs. 12.5 months (HR 0.58%, 95% CI: 0.38–0.88%).
Combination therapy with chemotherapy, radiotherapy and novel therapy
There has been significant interest in combining systemic therapy with immunotherapy in TNBC after single agent immunotherapy trials had poor response rates, especially in the second-line setting (Table 1). Combining immunotherapy with systemic is theoretically synergistic by multiple mechanisms. Systemic therapy can damage tumor cells, resulting in increased tumor antigen release (21). When combined with immunotherapy, there is theoretically increased numbers of activated T cells to provide an immune response against the neoplasm. Systemic therapy has also been shown to increase TILs during treatment, and patients with higher TILs treated with anti-PD1 therapy have superior ORR and OS in subgroup analysis of the KEYNOTE-119 trial (22). Combing immunotherapy with radiation therapy also has a hypothetical combined effect. Radiation therapy not only increases neo-antigen presentation, but also increases interferon-γ (INF-γ) which can improve T cell infiltration (23). While uncommon, the abscopal effect of local radiation minimizing or even eradicating metastases at distant sites is well described, and hypothesized to work through immune stimulation (24). Certain novel therapies also have theoretical benefit when combining with immunotherapy. Poly(ADP)-ribose polymerase (PARP) inhibitors in breast cancer gene (BRCA) mutated tumors results in accumulation of DNA damage, genomic instability, and up-regulated PD-L1 expression (25). Combining PARP inhibitors, or other targeted therapy which increase neo-antigen production have been a focus of combined therapy. Currently, immunotherapy combined with systemic therapy has become first-line treatment for PD-L1 positive mTNBC patients (26). A summary of combined chemoimmunotherapy trials is provided in Table 2, and immunotherapy with novel therapy agents in Table 3.
Table 1
| Trial (phase) | Treatment | Line of therapy | PD-L1 definition | No. total; PD-L1 pos. [%] | PD-L1 subgroup [n] | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|---|
| KEYNOTE-012 (phase 1b) (15) | Pembrolizumab | ≥2nd line | Any stromal or TC ≥1% | 32 | NA | 18.5 | 1.9 | 11.2 |
| 32 [100] | ||||||||
| NCT01375842 (phase 1b) (16) | Atezolizumab | Any | TIL ≥1% | 114 | Any [114] | 10 | 1.4 | 8.9 |
| 82% ≥2nd line | 91 [78] | PD-L1 ≥1% [91] | 12 | 1.4 | 10.0 | |||
| JAVELIN (phase 1b) (17) | Avelumab | Any | TC ≥1% or | 58 | Any [58] | 5.2 | 1.4 | 9.2 |
| 50% ≥2nd line | IC ≥10% | 33 [69]b ≥1% TC | ≥10% IC [9] | 22 | – | – | ||
| 9 [19]b ≥10% IC | ||||||||
| KEYNOTE-086 A (phase 2) (18) | Pembrolizumab | ≥2nd line | CPS ≥1% | 170 | Any [170] | 5.3 | 2.0 | 9.0 |
| 105 [62] | CPS ≥1% [102] | 5.7 | 2.0 | 8.8 | ||||
| KEYNOTE-086 B (phase 2) (19) | Pembrolizumab | 1st line | CPS ≥1% | 81 | CPS ≥1% [81] | 21 | 2.1 | 18 |
| 81 [100] | ||||||||
| KEYNOTE-119 (phase 3) (20) | Pembrolizumab vs. chemotherapya | 2nd–3rd | CPS ≥1% | 622 | Any [622] | 9.6 vs. 10.6 | 2.1 vs. 3.3† | 9.9 vs. 10.8 |
| 405 [65] | CPS ≥1% [405] | 12.3 vs. 9.4 | 2.1 vs. 3.1† | 10.7 vs. 10.2 | ||||
| CPS ≥10% [194] | 17.7 vs. 9.2 | 2.1 vs. 3.4 | 12.7 vs. 11.6 | |||||
| CPS ≥20% [109] | – | – | 14.9 vs. 12.5† |
a, physician’s choice (capecitabine, eribulin, gemcitabine or vinorelbine); b, percentage calculated from 48 patients with known PD-L1 status; †, results meet statistical significance. CPS, combined positive score (tumor cells, lymphocytes, and macrophages out of the total number of tumor cells × 100); mPFS, median progression free survival; mOS, median overall survival; NA, not applicable; ORR, overall response rate; PD-L1, programed cell death ligand 1; TC, tumor cell; TIL, tumor infiltrating leukocytes.
Table 2
| Trial (phase) | Treatment | Line of therapy | PD-L1 definition | No. total; PD-L1 pos. [%] | Subgroup [n] | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|---|
| NCT01633970 (phase 1b) (27) | Atezolizumab + nab-paclitaxel | 0–2 | IC ≥1% | 33 | Any [33] | 39.4 | 5.5 | 14.7 |
| 61% ≥2nd line | 12 [50]a | ≥1% IC [9] | 41.7 | 6.9 | 21.9 | |||
| ENHANCE-1 (phase 1b/2) (28) | Pembrolizumab + eribulin | 0–2 | CPS ≥1% | 167 | CPS <1% 1st line [31] | 16.1 | 3.5 | 15.2 |
| 60% ≥2nd line | 74 [50]a | CPS ≥1% 1st line [29] | 34.5 | 6.1 | 21.0 | |||
| CPS <1% 2nd-3rd line [101] | 18.2 | 3.9 | 15.5 | |||||
| CPS ≥1% 2nd-3rd line [45] | 24.4 | 4.1 | 14.0 | |||||
| TONIC (phase 2) (29) | Nivolumab + no induction, induction radiation, or induction chemotherapyb | 0–2 | IC ≥1% or TC ≥1% | 67 | Any [67] | 20 | 1.9 | NA |
| 76% ≥2nd line | IC 44 [63]a | No induction [12] | 17 | – | ||||
| TC 60 [86]a | Cisplatin [13] | 23 | – | |||||
| Cyclophosphamide [12] | 8 | – | ||||||
| Doxorubicin [17] | 35 | – | ||||||
| Radiation therapy [12] | 8 | – | ||||||
| NCT02730130 (phase 2) (30) | Pembrolizumab + radiation therapy | Any | ≥1% ME, and inflammation cells or positive stroma | 17 | Any | 17.6 | 2.6 | 8.3 |
| 88 ≥2nd line | 10 [67] | |||||||
| NCT02768701 (phase 2) (31) | Pembrolizumab + priming cyclophosphamide | ≥2nd line | NA | 40 | Any [40] | 21 | 1.8 | 6.3 |
| 29% ≥5th line | ||||||||
| KEYNOTE-355 (phase 3) (32) | Chemotherapyc ± pembrolizumab | 1st line | CPS ≥1% | 847 | Any [847] | NA | 7.5 vs. 5.6 | NA |
| 636 [75] | CPS ≥1% [636] | 7.5 vs. 5.6 | ||||||
| CPS ≥10% [323] | 9.7 vs. 5.6† | |||||||
| IMpassion-130 (phase 3) (26) | Nab-paclitaxel ± atezolizumab | 1st line | TIL ≥1% | 952 | Any [952] | 56.0 vs. 45.9† | 7.2 vs. 5.5 | 21.3 vs. 17.6 |
| 369 [38] | TIL ≥1% [369] | 58.9 vs. 42.6† | 7.5 vs. 5.0† | 25.0 vs. 15.5† | ||||
| IMpassion-131 (phase 3) (33) | Paclitaxel ± atezolizumab | 1st line | IC ≥1% | 652 | Any [652] | 53.6 vs. 47.5 | 5.7 vs. 5.6 | 19.2 vs. 22.8 |
| 292 [45] | IC ≥1% [292] | 63.4 vs. 55.4 | 6.0 vs. 5.6 | 22.1 vs. 28.3 |
a, percentage calculated from patients with known PD-L1 status; b, chemotherapy either (cisplatin, cyclophosphamide or doxorubicin); c, physicians’ choice of either nab-paclitaxel, paclitaxel or carboplatin and gemcitabine; †, results meet statistical significance. CPS, combined positive score (tumor cells, lymphocytes, and macrophages out of the total number of tumor cells × 100); IC, immune cell; ME, membranous cell; mPFS, median progression free survival; mOS, median overall survival; ORR, overall response rate; NA, not applicable; PD-L1, programed cell death ligand 1; TIL, tumor infiltrating leukocyte.
Table 3
| Trial (phase) | Treatment | Line of therapy | PD-L1 definition | No. total; PD-L1 pos. [%] | Subgroup [n] | ORR (%) | mPFS (months) | mOS (months) |
|---|---|---|---|---|---|---|---|---|
| NCT03800836 (phase 1b) (34) | Ipatasertib + atezolizumab + chemotherapya | 1st line | ≥1% IC† | 26 | Any [26] | 73 | NA | NA |
| 11 [58]b | PD-L1 positive [11] | 82 | ||||||
| MEDIOLA (phase 1/2) (25) | Durvalumab + olaparib | 0–2 | IC ≥1% or TC ≥1% | 17 | Any [17] | 58.8 | 4.9 | 20.5 |
| 74% ≥2nd line | NA | |||||||
| NCT03310957 (phase 1b/2) (35) | Pembrolizumab + LV | 1st line | NA | 26 | Any [26] | 54 | – | NA |
| TOPACIO (phase 2) (36) | Pembrolizumab + niraparib | Any | CPS ≥1% | 55 | Any [55] | 18 | 2.3 | NA |
| 40% ≥2nd line | 28 [60] | PD-L1 positive [28] | 32 | – | ||||
| BRCA mutant [15] | 47 | 8.3 | ||||||
| COLET (phase 2) (37) | Atezolizumab + cobimetinib + chemotherapya | 1st line | IC ≥1% | 63 | Paclitaxel any [32] | 34 | NA | NA |
| 31 [49] | Paclitaxel PD-L1 + [16] | 44 | ||||||
| Nab-paclitaxel any [31] | 29 | |||||||
| Nab-paclitaxel PD-L1 + [15] | 33 | |||||||
| ENCORE 602 (phase 2) (38) | Atezolizumab ± entinostat | ≥2nd line | NA | 81 | Any [81] | 10 vs. 2.4 | 1.7 vs. 1.5 | 9.8 vs. 12.4 |
| 31% ≥3rd line | ||||||||
| NCT03394287 (phase 2) (39) | Camrelizumab + apatinib | <3rd line | IC ≥1% or TC ≥1% | 40 | Any [40] | 32.5 | – | – |
| 75% 2nd or 3rd line | 14 [35] IC | Continuous treatmentc [30] | 43.3 | 3.7 | 8.1 | |||
| 13 [33] TC |
a, paclitaxel or nab-paclitaxel; b, percentage calculated from patients with known PD-L1 status; c, continuous afatinib cohort; †, determined through correspondence with the author. BRCA, breast cancer gene; CPS, combined positive score (tumor cells, lymphocytes, and macrophages out of the total number of tumor cells × 100); IC, immune cell; mPFS, median progression free survival; LV, lidiratuzumab vedotin; mOS, median overall survival; NA, not applicable; ORR, overall response rate; PD-L1, programed cell death ligand 1.
Phase 1 data
A multi-cohort phase I trial combining nab-paclitaxel with atezolizumab (NCT01633970) was tested in several solid tumors with advanced disease (27). In the TNBC cohort, patients had not been treated with more than two lines of previous systemic therapy for metastatic disease and were included regardless of PD-L1 status. Patients with ≥1% PD-L1 expression on immune cells were considered to be PD-L1 positive. Thirty-three patients with TNBC were treated, 13 in the first-line setting, and 20 in the second-line or later setting. For the entire population, the ORR was 39.4%. One (3.0%) patient had a CR, and there were 12 (36.4%) PRs. The disease control rate (DCR) was 51.5%. The ORR was higher in PD-L1 positive patients (41.4% vs. 33.3%) and was higher in the first-line setting (53.8% vs. 30%); however, these associations were not statistically significant. The mPFS for all patients was 5.5 months. The mPFS was longer in the first-line setting, 8.6 months compared to 5.1 months in the second-line or later setting, but statistical significance was not met. No significant difference in mPFS was found in the PD-L1 positive patients (6.9 vs. 5.1 months). The mOS for all patients was 14.7 months, 24.2 months in the first-line setting and 12.4 months in second-line or later. mOS was 11.4 months in PD-L1 negative population and 21.9 months in the PD-L1 positive population. No significant difference in mOS was found between first and later line treatment or PD-L1 populations.
Phase 2 data
The ENHANCE-1 trial (NCT02513472) was a phase 1b/2 trial with 167 patients with mTNBC who had received zero to two lines of systemic therapy in the metastatic setting (28). Patients were stratified to first or later line cohorts, regardless of PD-L1 status. PD-L1 positive was defined as CPS score ≥1%. All patients received eribulin in combination with pembrolizumab. Sixty-six patients were evaluated in the first-line setting, and 101 patients were evaluated in second or later line. The ORR for the entire cohort was 25.8%, with numerically higher response rates seen in first-line and PD-L1 positive patients. The ORRs were 34.5% and 16.1% for PD-L1 positive and negative patients treated in the first-line setting. In the second or later-line setting, the ORR was 24.4% and 18.2% for PD-L1 positive and negative patients, respectively. In the first-line setting, the mPFS was 6.1 and 3.5 months, and the mOS was 21.0 and 15.2 months for PD-L1 positive and negative patients. The mPFS for later line patients was 4.1 and 3.9 months for PD-L1 positive and negative patients, and the mOS was 14.0 and 15.5 months. There was no statistical difference for ORR, mPFS, and mOS between the PD-L1 positive and negative groups for either patient cohort.
In the TONIC trial (NCT02499367), patients with mTNBC previously treated with less than two lines of systemic therapy in the metastatic setting were randomized to 1 of 5 different 2-week induction treatment arms (29). The induction treatment arms were: three fractions of 8,000 centigrays (cGy) radiation to a metastatic lesion, doxorubicin 15 mg weekly for two doses, cisplatin 40 mg /m2 weekly for two doses, cyclophosphamide 50 mg oral daily for 2 weeks, and no induction treatment. After the 2-week induction period, all patients received nivolumab. Of the 66 patients enrolled, 23% were evaluated in the first-line setting, and 77% were in the second-line or later setting. The ORR was 20% for the entire cohort. There were 2 (3%) CRs and 11 (17%) PRs. The CRs were in the doxorubicin and cisplatin patients. The ORR was highest in the doxorubicin and cisplatin treatment arms at 35% and 23%, respectively. The ORR for radiation and cyclophosphamide was 8% and 17% in the no induction arm. The ORR for first-line patients was 33% and 16% in the second or later lines. The mPFS was 1.9 months in the entire cohort, and mOS data for the entire cohort and individual treatment arms was not published.
The combination of immunotherapy along with radiation therapy was evaluated in a phase 2 trial (NCT02730130) (30). Patients with mTNBC were treated with 3,000 cGy over five fractions in combination with pembrolizumab. Patients could be previously treated for mTNBC or be treatment naïve, and were included regardless of PD-L1 status. Pembrolizumab was started within 3 days of first radiation therapy and continued until progression. Of the 17 patients enrolled in the study, the ORR was 17.6%, with all responding patients having a CR. The mPFS was 2.6 months, and the mOS was 8.3 months. There was no association between PD-L1 status and ORR. The CRs were found to have durable responses at 18, 20 and 108 weeks.
The combination of pembrolizumab and cyclophosphamide was tested in the second-line setting in a phase II clinical trial (NCT02768701) (31). All patients had mTNBC, with at least one line of systemic treatment in the metastatic setting. Patients received only one dose of cyclophosphamide on day 1, received pembrolizumab on day 2, and continued pembrolizumab every 3 weeks. Preliminary results presented showed 40 patients had been enrolled, and the ORR rate was 21% with no CRs. The median pPFS and mOS was 1.8 and 6.3 months, respectively.
Phase 3 data
The KEYNOTE-355 (NCT02819518) is a phase 3 clinical trial which randomized patients to either placebo or pembrolizumab combined with chemotherapy (32). Three different chemotherapy regimens of either nab-paclitaxel, paclitaxel or carboplatin and gemcitabine could be used depending on the treating physician’s choice. A total of 847 patients were enrolled, without previous first-line treatment for mTNBC. Patients could have any PD-L1 status, with PD-L1 positive defined as a CPS of ≥1%. Initial presented data showed the pre-specified group of CPS ≥10% had mPFS of 9.7 vs. 5.6 months for the pembrolizumab and the placebo groups, which was statistically significant. For patients with a CPS ≥1%, pembrolizumab extended mPFS numerically, to 7.6 months from 5.6 months with placebo, but this did not meet the pre-specified statistically significant boundary. ORR were not presented, and the mOS data is ongoing.
The only currently published phase 3 trial of combination immunotherapy and chemotherapy is the IMpassion 130 trial (NCT02425891) (26). This trial randomized patients with advanced unresectable or mTNBC to nab-paclitaxel and placebo or nab-paclitaxel and atezolizumab for first-line treatment. There were 902 patients enrolled in the study, 369 (40.9%) were PD-L1 positive (≥1% PD-L1 expression of TIL). The ORR in the entire study was 56.0% vs. 45.9% in the atezolizumab group compared to placebo (HR 1.52; 95% CI: 1.16–1.97). In the immunotherapy arm, there were 7.1% of patients with a CR compared to 1.6% in the placebo arm. In the PD-L1 positive subgroup, the ORR was 58.9% vs. 42.6% (HR 1.96; 95% CI: 1.29–2.98). 10.3% of PD-L1 positive patients had a CR, compared to 1.1% of patients in the immunotherapy and placebo groups, respectively. Median PFS was improved from 5.5 to 7.2 months in the entire cohort (HR 0.80; 95% CI: 0.69–0.92). In the PD-L1 positive patients, mPFS with immunotherapy was 7.5 months compared to 5.0 months with placebo (HR 0.62; 95% CI: 0.49–0.78). The mOS had a trend to improved survival in the combined immunotherapy arm, 21.6 vs. 17.6 months; however, it was not statistically significant (HR 0.84; 95% CI: 0.69–1.02). Given that the overall cohort OS was not significant, subgroups were not able to be formally assessed. The PD-L1 positive group, when evaluated using Kaplan-Meier curves, showed an improved OS of 25.0 vs. 15.5 months with combined therapy (HR 0.62; 95% CI: 0.45–0.86). In the PD-L1 positive subgroups, the duration of response was 8.5 months vs. 5.5 months with combined therapy compared to chemotherapy (HR 0.60; 95% CI: 0.43–0.86).
The Impassion 131 trial compared the combination of paclitaxel and atezolizumab to paclitaxel alone and was recently presented but is not currently published (NCT03125902) (33). In this trial, 651 patients with mTNBC were treated in the first-line setting, regardless of PD-L1 status. There were 292 PD-L1 positive (immune cells ≥1% PD-L1 expression) patients. The entire cohort ORR was 53.6% in the atezolizumab arm vs. 47.5% in the placebo arm, and was 63.4% vs. 55.4% in the PD-L1 positive group, respectively. There was no statistical difference in ORR regardless of PD-L1 status. In the atezolizumab group the mPFS was 5.7 vs. 5.6 months in the placebo group, and for PD-L1 patients’ mPFS was 6.0 and 5.7 months. No statistical difference was found in mPFS. There was a trend towards worsened survival with atezolizumab for mOS; however, there was no statistical difference in mOS regardless of PD-L1. The mOS was 19.2 vs. 22.8 months in the entire cohort, and was 22.1 vs. 28.3 months for PD-L1 positive patients.
Novel targeted therapy combined with immunotherapy
Phase 1
Protein kinase B, also known as AKT, is part of an important signaling pathway in the PI3K/AKT/mTOR pathway which is frequently abnormal in breast cancer (40). The AKT inhibitor ipatasertib was combined with atezolizumab, and paclitaxel or nab-paclitaxel in a phase 1b clinical trial (NCT03800836) (34). Patients were mTNBC without prior therapy. The initial analysis was presented at American Association for Cancer Research Annual Meeting in 2019. Twenty-six patients had a median follow-up time of 6.1 months. The ORR was 73% with an ORR of 82% and 75% in PD-L1 positive and negative patients, respectively (Table 3).
Phase 2
The PARP inhibitor olaparib was tested in combination with durvalumab (PD-L1 mAB) in the phase 1/2 MEDIOLA trial (NCT02734004) (25). All patients evaluated had MBC, had a deleterious germline BRCA1 or BRCA2 mutation and were HER2 negative. Patients had between zero and two previous lines of systemic therapy for metastatic disease, were previously treated with an anthracycline and taxane, and were included regardless of PD-L1 status. Thirty-four patients participated in the trial, 30 of which were included in the treatment analysis outcomes. Of the 17 patients with mTNBC, the ORR was 58.8%, and the mPFS was 4.9 months. The mOS for the mTNBC cohort was 20.5 months, and the duration of response was 7.2 months.
The zinc transporter LIV-1, is known to be upregulated in TNBC and can be found in around 65% of TNBC (41,42). The anti-body drug conjugate ladiratuzumab vedotin is a LIV-1 antibody that selectively delivers the highly cytotoxic agent monomethyl auristatin E (MMAE) via a protease-cleavable linker to LIV-1 (35). In a phase Ib/2 clinical trial (NCT03310957), the antibody conjugate was tested in combination with pembrolizumab in first-line mTNBC patients (35). Patients were selected regardless of PD-L1 or LIV-1 status. In the initial analysis presented, 51 patients received treatment, 26 of which had measurable outcomes. The ORR is 54%, and survival data is still pending.
The combination of niraparib (an oral PARP inhibitor) and pembrolizumab was evaluated in the phase 2 TOPACIO trial (NCT02657889) (36). Patients had measurable mTNBC, had received zero to two lines of systemic therapy in the metastatic setting, and were included regardless of PD-L1 status or BRCA1/BRAC2 mutational status. Patients with a CPS ≥1% were classified as being PD-L1 positive. Fifty-five patients were enrolled in the study. The ORR for the efficacy-evaluable population was 21%, and the DCR was 49%. Nine (11%) of patients had a CR. In the 15 patients who had BRCA mutations, the ORR was 47%, the CR was 13%, the DCR was 80%, and the mPFS was 8.3 months. The response rates were numerically less in the BRCA wildtype group with the ORR being 11%, CR 11%, and DCR being 33%. The mPFS in the BRCA wildtype group was 2.1 months, and was not statistically different from the mPFS in the BRCA mutant group. Twenty-eight patients were confirmed to be PD-L1 positive, and the PD-L1 positive ORR was 32% compared to 8% in the PD-L1 negative group. The mOS data was not mature at the time of analysis.
The combination of immunochemotherapy with the mitogen activated protein kinase (MEK) inhibitor cobimetinib was evaluated in the phase 2 COLET study (NCT02322814) (37). Patients with mTNBC with no prior systemic therapy in the metastatic setting were randomized to either nab-paclitaxel or paclitaxel, in combination with atezolizumab and oral cobimetinib. Sixty-three patients were randomized to the paclitaxel group and 62 were randomized to the nab-paclitaxel group. The ORR was 34% in the paclitaxel arm, and 29% in the nab-paclitaxel arm. There were no CRs in either treatment arm. The mPFS and mOS data was not mature at this time.
The oral histone deacetylase inhibitor entinostat has been shown to prevent metastasis of TNBC by reversing the epigenetic repression of E-cadherin (43). This prevents the epithelial to mesenchymal transition required for metastasis. In the phase II trial ENCORE 602, 81 patients with mTNBC were randomized to atezolizumab and entinostat or atezolizumab and placebo (NCT02708680) (38). Patients had one or two lines of prior therapy in the advanced setting, and were immunotherapy naive. The results initial data presented showed the ORR were not statistically improved with entinostat compared to placebo (10.0% vs. 2.4%). The mPFS (1.7 vs. 1.5 months) and mOS (9.9 vs. 12.4 months) were also not significantly different between treatment arms.
The fully humanized PD-1 mAB camrelizumab, was combined with the oral vascular endothelial growth factor (VEGF) inhibitor apatinib in an open label phase II clinical trial (NCT03394287) (39). Patients had mTNBC and less than three lines of systemic therapy in the metastatic setting. PD-L1 positive was defined as ≥1% for immune cells or tumor cells. Patients were initially randomized to either camrelizumab with intermittent apatinib (daily for 7 days) or continuous apatinib (daily for 14 days) in a 21-day cycle. The majority of patients (75%) had received at least one line of therapy in the metastatic setting. Given no patient in the intermittent dosing had a PR, in the second phase of the trial, patients were all given continuous apatinib. A total of 40 patients were enrolled in the study, 30 in the continuous cohort. The ORR for the entire cohort was 32.5%, and 43.3% for the continuous dosing cohort. An additional 25% and 20% of patients had SD in the entire cohort and continuous treatment cohort, respectively. There were no CR in the trial. The mPFS was 3.7 months and the mOS was 8.1 months in the continuous treatment arm. PD-L1 expression did not correlate with ORR or mPFS.
Discussion
Monotherapy
While the initial phase 1 trials of PD-1 and PD-L1 monotherapy suggested promise, the phase 2 and phase 3 data has been largely disappointing. The mPFS and mOS outcomes with immune monotherapy have not improved historical landmarks established with chemotherapy. The response rates and OS in first-line setting, appear to be better than in the second-line setting, but only one dedicated first-line monotherapy trial has been performed (Table 1). There is no approved use of PD-1/PD-L1 monotherapy for mTNBC; however, the trials provide some insight into subgroups of patient responders and possible prognostic biomarkers (Figure 2).
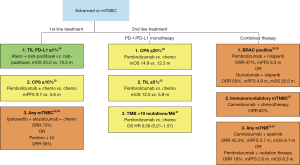
In the KEYNOTE-119 trial, patients with a CPS ≥20% had a modest 2.6-month improvement in mOS, but only represent around 18% of the enrolled patients (20). Retrospective analysis showed patients with TILs <5% had a mOS of 5.9 months compared to 12.5 months for patients with TILs ≥5% (22). Two hundred and fifty-three of 601 patients had FoundationOne CDX testing to evaluate tumor mutational burden (TMB) (44). Around 10% of patients were found to have ≥10 mutations per megabase (Mb). The ORR were similar between regardless of mutational burden, but those with TMB ≥10 per Mb had a HR of 0.58 (0.21–1.57) for OS when treated with pembrolizumab. Now that combination atezolizumab with nab-paclitaxel has become standard of care in PD-L1 positive mTNBC, the role of PD1/PD-L1 monotherapy is unlikely to play a significant role in the future.
Combined therapy
With the poor outcomes of single agent immunotherapy, there has been a focus on combining systemic treatment with immunotherapy to increase neo-antigen release, increase TILs, and provide synergistic immune responses. The results of the IMpassion-130 trial with combination atezolizumab and nab-paclitaxel has changed the standard of care for first-line mTNBC treatment for patients with PD-L1 ≥1% of TILs (26). Although the overall population did not have an improved survival, there was almost a 10-month median survival benefit for PD-L1 positive patients. While this has drastically improved historical outcomes, the durability of outcomes so far has been disappointing. Patients with other malignancies, such as melanoma, lung cancer, and renal cancer often have prolonged stability of their disease when they respond to treatment (7-9). Examining the IMpassion-130 PD-L1 positive Kaplan-Meier curves for OS shows a separation of the curves, but no prolonged tail in the immunotherapy group (26). It is possible that, with time, we will see continued stability of disease for some patients with responses. Long-term stability of disease was seen in phase 1 atezolizumab monotherapy trial and KEYNOTE-086 trials (16,18,19). In the phase 1 atezolizumab trial, 9 of the 15 (60%) patients who experienced a response to treatment had an ongoing response, with response times up to 45 months (16).
It is important to note that improved outcomes with combination therapy have not yet been duplicated in mTNBC. The IMpassion-131 study did not show any improvement in ORR or mPFS with atezolizumab (33). Analysis for survival actually showed a trend towards worsened survival in the entire cohort and the PD-L1 positive population. Possible explanations for the negative trial might be due to the different mechanism of action of nab-paclitaxel compared to paclitaxel, that treatment with weekly paclitaxel requires dexamethasone, or lack of a synergistic effect with PD-1 therapy combined with paclitaxel (45). Paclitaxel is highly lipophilic and requires a solvent to be formulated, while nab-paclitaxel consists of a colloidal suspension of albumin-bound paclitaxel nanoparticles, negating the need for a solvent. While this removes the potential for suspension related hypersensitivity reactions and the need for pre-treatment with steroids, it also provides nab-paclitaxel with different pharmacologic properties (45). Nab-paclitaxel has linear pharmacokinetics, and a higher maximum tolerated dose. The albumin of nab-paclitaxel has a natural affinity for the gp60/caveolin-1 pathway, providing a 33% higher intratumoral drug concentration and four-fold lower elimination rate than solvent paclitaxel.
Giving steroids along with immunotherapy has always been a concern for decreasing effectiveness, and some argue it may have played a role in the negative IMpassion-131 trial. Outcomes of patients with melanoma and non-small cell lung cancer given steroids for immune-related toxicities have not shown worsened OS (46,47). Non-small cell lung cancer treated with first-line cisplatin, pemetrexed and pembrolizumab also received dexamethasone, and had an improved mOS in the KEYNOTE-189 trial (48). Regardless of potential reasons for the negative IMpassion-131 trial, it has cast enough doubt on the benefit of nab-paclitaxel combined with atezolizumab that the Federal Drug Agency released a statement that continued approval may be contingent upon proven benefit in additional trials (49).
Side effects
While immunotherapy is typically better tolerated than chemotherapy, the side effects are diverse and can affect any organ system (50). The most common side effects are rash, fatigue, nausea, arthralgia with most cases being mild, but patients can have severe complications such as hypophysitis, cardiomyopathy, pneumonitis, GI perforation, encephalitis or Guillain-Barre syndrome to name only a few (51). In the KEYNOTE-119 trial, immune monotherapy had ≥ grade 3 treatment related adverse events in 14% of patients vs. 36% with chemotherapy (20). When atezolizumab was combined with chemotherapy, treatment related adverse events ≥ grade 3 occurred in 40.3% in the immunotherapy arm with three related treatment deaths, compared to 30.3% in the placebo arm, with one related treatment death (26). The three deaths in the immunotherapy arm were autoimmune hepatitis, mucosal inflammation and septic shock. Only 6.4% of patients needed to discontinue atezolizumab, compared to 1.4% of placebo matched patients, however, 15.9% vs. 8.2% of patients needed to discontinue nab-paclitaxel in the immunotherapy vs. placebo groups respectively. The most common adverse events were similar between treatment arms; however, nausea, cough, pyrexia, neutropenia and hypothyroidism were all five percent higher in the immunotherapy arm. Combining novel therapies with immunotherapy will have different side effects profiles, and require treating physicians to stay vigilant for a breadth of side effects.
Biomarkers
Monotherapy and combined therapy trials have shown the need for prognostic biomarkers in mTNBC beyond PD-L1 to see if certain patients will obtain clinically significant benefit. Monotherapy trials have reported that TMB, TILs, and different CPS thresholds may be useful biomarkers (Figure 2). It is important to realize that TNBC is comprised of a heterogeneous group of patients. Recently, it has been reported that TNBC can be further sub-divided into at least 6 different subtypes (52). Further evaluation of outcomes for mTNBC subgroups evaluating different gene expression profiles may explain variable outcomes.
In the FUTURE trial, patients with mTNBC were evaluated for tumor genomic biomarkers and classified into further subtypes. Sixty patients were evaluated, and 19 were classified as having immunomodulatory (IM) mTNBC (53). Despite being treated with a median of three prior lines of therapy in the metastatic setting, the ORR for IM mTNBC patients with nab-paclitaxel and anti-PD-1 immunotherapy was 63%, much higher than previous second-line trials (Table 2). A retrospective analysis of 62 patients treated with either single agent immunotherapy or combined immunotherapy and systemic therapy, analyzed outcomes in relation to tumor genomic features (54). The study found patients with the phosphatase and tensin homolog (PTEN) gene had significantly lower response rates, significantly shorter mPFS (2.3 vs. 6.1 months) and significantly shorter mOS (9.7 vs. 20.5 months).
Future directions
Combining immunotherapy with multiple systemic therapies or novel systemic therapies appears to be the future direction of immunotherapy for mTNBC. Hopefully the high response rates seen in Table 3 will provide survival benefits. Certain populations like PD-L1 positive, BRCA positive patients, patients with high TMB, TILs and certain genetic populations may benefit more than other groups (Figure 2). With the increased speed and decrease in price of next generation sequencing, we are likely to see more personalized medicine in mTNBC.
The idea that PD-1 or PD-L1 immunotherapy in mTNBC can be used as maintenance therapy may develop further in the future as well. Patients on first- or second-line chemotherapy, with SD or better during treatment and who lacked an actionable mutation were randomized to either maintenance durvalumab or chemotherapy (55). The patients with mTNBC treated with imunotherapy were found to have an improved OS of 21 vs. 14 months HR 0.54 (0.30–0.97). Other non-immunotherapy agents continue to be studied as well. The novel antibody conjugate sacituzumab govitecan, recently presented had a large survival benefit in the second-line or later setting for mTNBC (56).
Conclusions
Immunotherapy for the treatment of mTNBC is a rapidly evolving field, with many new and ongoing combination trials. Combination atezolizumab and nab-paclitaxel is now a standard first-line therapy for mTNBC treatment-naive patients with PD-L1 ≥1% of TIL; however, continued approval may require further proven benefit in additional trials. Immunotherapy in mTNBC requires further evaluation with appropriate biomarkers, and phase 3 trials to see if combination therapy with immunotherapy will provide further breakthroughs much needed and eagerly awaited in mTNBC.
Acknowledgments
We would like to thank all the medical oncologists at the Tom Baker Cancer Centre who informally discussed their individual thoughts on immunotherapy in TNBC. Thank you to Dr. Omar Khan, Dr. Morris, and Dr. Doug Stewart for expressing their interpretations in clinical trial data.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Jacques Raphael for the series “Management of Triple Negative Breast Cancer” published in Precision Cancer Medicine. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at: http://dx.doi.org/10.21037/pcm-20-58
Peer Review File: Available at http://dx.doi.org/10.21037/pcm-20-58
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/pcm-20-58). The series “Management of Triple Negative Breast Cancer” was commissioned by the editorial office without any funding or sponsorship. NAN reports grants from Pfizer, personal fees from Pfizer, personal fees from Novartis, personal fees from Roche, personal fees from Eli Lily, personal fees from Astra Zeneca, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects for the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429-34. [Crossref] [PubMed]
- Carey LA, Perou C, Livasy C, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492-502. [Crossref] [PubMed]
- Li X, Yang J, Peng L, et al. Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat 2017;161:279-87. [Crossref] [PubMed]
- Cardoso F, Senkus E, Costa A, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann Oncol 2018;29:1634-57. [Crossref] [PubMed]
- Kassam F, Enright K, Dent R, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer 2009;9:29-33. [Crossref] [PubMed]
- Yardley DA, Coleman R, Conte P, et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol 2018;29:1763-70. [Crossref] [PubMed]
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535-46. [Crossref] [PubMed]
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378:1277-90. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Liu Z, Li M, Jiang Z, et al. A comprehensive immunologic portrait of triple-negative breast cancer. Transl Oncol 2018;11:311-29. [Crossref] [PubMed]
- Kwa M, Adams S. Prognostic and predictive value of tumor infiltrating lymphocytes in breast cancer. Curr Breast Cancer Rep 2016;8:1-13. [Crossref]
- Cimino-Mathews A, Thompson E, Taube JM, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum Pathol 2016;47:52-63. [Crossref] [PubMed]
- Sabatier R, Finetti P, Mamessier E, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 2015;6:5449-64. [Crossref] [PubMed]
- Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase 1b KEYNOTE-012 study. J Clin Oncol 2016;34:2460-67. [Crossref] [PubMed]
- Emens LA, Cruz C, Eder JP, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol 2019;5:74-82. [Crossref] [PubMed]
- Dirix LY, Takacs I, Jerusalem G, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 2018;167:671-86. [Crossref] [PubMed]
- Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:397-404. [Crossref] [PubMed]
- Adams S, Loi S, Toppmeyer D, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 2019;30:405-11. [Crossref] [PubMed]
- Cortés J, Lipatov O, Im SA, et al. LBA21 - KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Ann Oncol 2019;30:v851-934. [Crossref]
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1-10. [Crossref] [PubMed]
- Loi S, Winer E, Lipatov O, et al. Relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treatment metastatic triple-negative breast cancer (mTNBC). Cancer Res 2020;80:abstr PD5-03.
- Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831-8. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Domchek SM, Postel-Vinay S, Im S, et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicenter, phase 1/2, basket study. Lancet Oncol 2020;21:1155-64. [Crossref] [PubMed]
- Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108-21. [Crossref] [PubMed]
- Adams S, Diamond JR, Hamilton E, et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol 2019;5:334-42. [Crossref] [PubMed]
- Tolaney SM, Kalinsky K, Kaklamani VG, et al. A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). J Clin Oncol 2020;38:abstr 1015.
- Voorwerk L, Slagter M, Horlings HM, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med 2019;25:920-8. [Crossref] [PubMed]
- Ho AY, Barker CA, Arnold BB, et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer 2020;126:850-60. [Crossref] [PubMed]
- Anders CK, Moore D, Sambade M, et al. LCCC 1525: A phase II study of a priming dose of cyclophosphamide prior to pembrolizumab to treat metastatic triple negative breast cancer (mTNBC). AACR 2019;79:abstr P2-09-05.
- Cortes J, Cescon DW, Rugo S, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol 2020;38:abstr 1000.
- Miles DW, Gligorov J, Andre F, et al. LBA15 - Primary results from IMpassion131, a double-blind placebo-controlled randomised phase III trial of first-line paclitaxel (PAC) ± atezolizumab (atezo) for unresectable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol 2020;31:S1142-215. [Crossref]
- Schmid P, Doirat D, Savas P, et al. Phase Ib study evaluating a triplet combination of ipatasertib (IPAT), atezolizumab (atezo), and paclitaxel (PAC) or nab-PAC as first-line (1L) therapy for locally advanced/metastatic triple-negative breast cancer (TNBC). Cancer Res 2019;79:abstr CT049.
- Han H, Diab S, Alemany C, et al. Abstract PD1-06: Open label phase 1b/2 study of ladiratuzumab vedotin in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. Cancer Res 2020;80:abstr PD1-06.
- Vinayak S, Tolaney SM, Schwartzberg L, et al. Open-label Clinical Trial of Niraparib Combined with Pembrolizumab for Treatment of Advanced or Metastatic Triple-Negative Breast Cancer. JAMA Oncol 2019;5:1132-40. [Crossref] [PubMed]
- Brufsky A, Kim SB, Zvirbule Z, et al. Phase II COLET study: atezolizumab (A) + cobimetinib (C) + paclitaxel (P)/nab-paclitaxel (nP) as first-line (1L) treatment (tx) for patients (pts) with locally advanced or metastatic triple-negative breast cancer (mTNBC). J Clin Oncol 2019;37:abstr 1013.
- O'Shaughnessy J, Moroose R, Babu S, et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J Clin Oncol 2020;38:abstr 1014.
- Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer 2020;8:e000696. [Crossref] [PubMed]
- Costa RL, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat 2018;169:397-406. [Crossref] [PubMed]
- Taylor KM, Morgan H, Johnson A, et al. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J 2003;375:51-9. [Crossref] [PubMed]
- Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody-drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther 2014;13:2991-3000. [Crossref] [PubMed]
- Shah P, Gau Y, Sabnis G. Histone deacetylase inhibitor entinostat reverses epithelial to mesenchymal transition of breast cancer cells by reversing the repression of E-cadherin. Breast Cancer Res Treat 2014;143:99-111. [Crossref] [PubMed]
- Winer E, Lipatov O, Im S, et al. Association of tumor mutational burden (TMB) and clinical outcomes with pembrolizumab (pembro) versus chemotherapy (chemo) in patients with metastatic triple-negative breast cancer (mTNBC) from KEYNOTE-119 J Clin Oncol;2020;38:abstr 1013.
- Schettini F, Giuliano M, De Placido S, et al. Nab-paclitaxel for the treatment of triple-negative breast cancer: Rationale, clinical data and future perspectives. Cancer Treat Rev 2016;50:129-41. [Crossref] [PubMed]
- Suo A, Chan Y, Beaulieu C, et al. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncologist 2020;25:438-46. [Crossref] [PubMed]
- Horvat TZ, Adel NG, Dang TO, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33:3193-8. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- FDA alerts health care professionals and oncology clinical investigators about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer. News release. FDA. September 8, 2020. Accessed September 10, 2020. Available online: https://bit.ly/328EFvy
- Brigden M, Humphreys M, Imbulgoda A. Delivering immuno-oncology therapies in the community oncology setting: Introduction of anti-PD-1 therapy into two community oncology programs. Oncol exch 2016;15:10-4.
- Su C, Wang H, Liu Y, et al. Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-small Cell Lung Cancer. Front Oncol 2020;10:554313. [Crossref] [PubMed]
- Lehmann BD, Bauer J, Chen X, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest 2011;121:2750-67. [Crossref] [PubMed]
- Jiang YZ, Liu Y, Xiao Y, et al. Molecular subtyping and genomic profiling expand precision medicine in refractory metastatic triple-negative breast cancer: the FUTURE trial. Cell Res 2021;31:178-86. [Crossref] [PubMed]
- Barroso-Sousa R, Keenan TE, Pernas S, et al. Tumor Mutational Burden and PTEN Alterations as Molecular Correlates of Response to PD-1/L1 Blockade in Metastatic Triple-Negative Breast Cancer. Clin Cancer Res 2020;26:2565-72. [Crossref] [PubMed]
- Dalenc F, Garberis I, Fillteron T, et al. Durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: Results from phase II randomized trial SAFIR02-IMMUNO. Cancer Res 2020;80:abstr GS3-02.
- Bardia A, Tolaney S, Loirat D, et al. LBA17 - ASCENT: A randomized phase III study of sacituzumab govitecan (SG) vs treatment of physician’s choice (TPC) in patients (pts) with previously treated metastatic triple-negative breast cancer (mTNBC). Ann Oncol 2020;31:S1142-215. [Crossref]
Cite this article as: Koczka KW, Nixon NA. The role of immunotherapy in metastatic triple negative breast cancer: a narrative review of the current clinical trials. Precis Cancer Med 2021;4:1.

