
Precision oncology in EGFR positive non-small cell lung cancer: breaking the 10-year barrier—a case report
Introduction
Lung cancer (LC) is associated with most cancer-related deaths in both sexes worldwide (1,2). The total number of deaths attributed to LC is greater than from colon, prostate, and breast cancer combined. This dismal outcome LC is due, in part to the fact that more than half of the patients, about 55%, presented with metastatic LC at the time of diagnosis. LCs kill more people in Latin America (LATAM) than any other malignancy. According to the International Agency for Research on Cancer, in 2012, just more than 60,000 people died of LC in the LATAM region. This represents >10,000 more lives lost than the next most lethal cancer and approximately 11–12% of all neoplasm deaths (2,3). According to GLOBOCAN, in Colombia LC is the fourth most incident cancer and the second in mortality (3). Currently, it is reported that LC incidence is rising in women; this increased number of cases has been attributed to some hormonal factors and genetic variants (2). Moreover, it is known that cigarette smoking, exposure to environmental and occupational factors play an essential role in LC development. The risk of LC development because of tobacco use is 1 in 17 women and 1 in 14 men (4). However, ~20% of LC cases occur in never smokers with a higher incidence in the female population (2).
There are two main LC subtypes, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), accounting for 15% and 85% of the cases. Based on the histological characteristics, the World Health Organization (WHO) classified NSCLC into three types: squamous cell carcinoma, large cell neuroendocrine carcinoma, and adenocarcinoma. Being adenocarcinoma the most common type (~60% of all cases) (5,6). NSCLC typically presents in an advanced stage and has a poor outcome. Median overall survival (OS) for metastatic NSCLC patients is about 4–5 months with supportive care alone. For those cases that receive supportive care in conjunction with platinum-based chemotherapy, historically, median OS has been 8–12 months. For decades, multiple trials have compared different chemotherapy regimens and resulted in marginal improvements in OS (7). Research examining treatment benefits of chemotherapy has plateaued. In 2002, the Eastern Cooperative Oncology Group (ECOG) published results of a randomized phase III trial comparing four platinum-based doublets in first-line metastatic NSCLC. The study demonstrated no difference in OS among the different treatment regimens (8). Furthermore, 10% of NSCLC patients have brain metastases (BM) at the diagnosis, and 25–40% of NSCLC cases will develop BM, being adenocarcinoma more than half of all BM cases (9,10). These patients commonly present complications with disabling neurological symptoms, resulting in a poor quality of life (11). Notably, patients with BM have poor survival, 1 month without treatment, 2 months with glucocorticoid therapy, 2.4–4.8 months with whole-brain radiation therapy (WBRT) and an OS from 7.4–10 months using platinum combined chemotherapy (9,10). However, in patients with BM, TKI therapy improved progression-free survival (PFS) of 6.6 to 15.2 months and OS of 12.9 to 18.9 months (9).
A major advancement in the treatment of metastatic NSCLC came with the identification of specific driver mutations and the development of targeted therapy. Although, the subset of patients with actionable mutations is small, PFS significantly increased in patients treated with targeted therapy compared to those treated with chemotherapy. The response rate range is 50% to 80% for patients who harbored EGFR, ALK, ROS1, and BRAF mutations and received targeted therapy. OS was increased to between 18 and 38.6 months (6,12).
Current evidence has strongly suggested that ethnicity might be a risk factor for EGFR-mutant LC, with mutations present in 15% of patients with NSCLC in Western population and rising to 35% in Asian population. Analyses of EGFR mutation, frequencies have showed varying rates in LATAM countries (approximately 15% in Argentina; 20–25% in Brazil; 25–35% in Mexico, Costa Rica, and Colombia; and 55% in Peru) (13). These observations suggest that somatic mutation frequency in EGFR in LC could be associated with genetic ancestry (14).
A plethora of robust clinical evidence, has provided the proof of concept for targeting EGFR mutation-positive NSCLC with TKIs, and data showing that epithelial growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are superior to chemotherapy in this setting have supported their use as first-line standard of care. As of today, gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib are all FDA-approved first-line treatment of patients with metastatic NSCLC whose tumors have EGFR exon 19 deletions or exon 21 L858R mutations. Afatinib was also active in NSCLC tumors that harbored certain types of uncommon EGFR mutations, especially Gly719Xaa, Leu861Gln, and Ser768Ile (15). Recently, osimertinib, originally developed to treat T790M-mediated resistance to first-generation TKIs (16), became a first-line option, on the basis of findings from the randomized phase III FLAURA study (17). In which third-generation TKI demonstrated improved PFS (median, 18.9 vs. 10.2 months) and OS (median, 38.6 vs. 31.8 months) compared with first-generation TKIs, and improved control of central nervous system (CNS) metastases (18,19). Because osimertinib has moved to the front-line setting, we are now increasingly faced with the challenge of selecting the optimal sequence of treatment.
Diversity in resistance patterns in EGFR positive NSCLC ranges from mutations in TK domain (T790M or C797X), to MET and EGFR amplification, T790M lost, translocations in RET, FGFR3, ALK, BRAF, and bypass mutations in BRAF, KRAS, JAK and NF1 (6,12). Histologic transformations have been reported in up to 15% of patients with disease progressing on first-line osimertinib and highlight the critical role of tissue biopsy at progression (20). Small-cell transformation, has been well described in EGFR-mutant cancers and occurs in approximately 3–5% of patients whose cancer progresses on first- and second-generation EGFR-TKIs (21,22).
Despite of encouraging survival results, evidence of long-term survival in NSCLC patients treated with target therapy is limited. Data on 5-year survival, that serve as traditional indicator for cure in many cancer types, in NSCLC is in an estimate of 5–20% in the available results (7). Consequentially, data from survival for more than 10 years are rare and long-term survival in NSCLC treated with targeted therapy remains uncertain (22). Hence, we report a case of a metastatic EGFR-mutated NSCLC patient who went through multiple medical treatments, achieving more than 10 years survival.
Novel points of the case report include:
- After years of standard care prescribed to cancer patients without any selection except the primary site and histology of the tumor, the era of precision medicine has revolutionized LC care.
- Targeted therapy inhibiting specific actionable driver genes has resulting in a significant improvement in response rate and disease control.
- Evaluating the molecular profile using next-generation sequencing (NGS) completely changed the diagnosis, prognosis, and management of this case considering the use of information to dynamically adapt treatment according to resistance patterns.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-53).
Case presentation
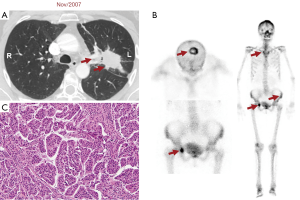
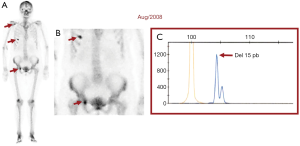
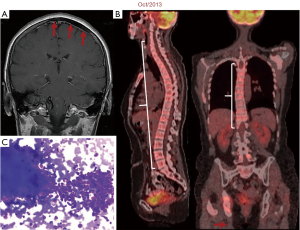
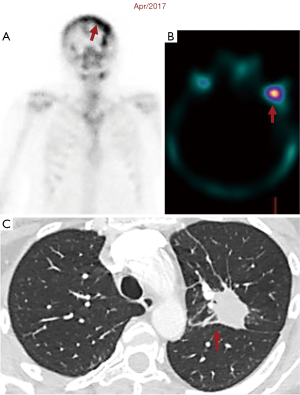
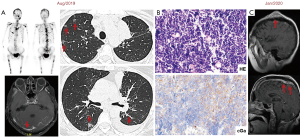
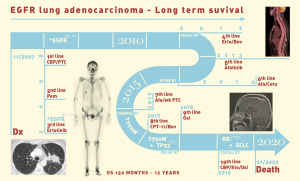
Twelve years ago, our patient was a 53-year-old never smoker woman of Hispanic origin that debuted with dry cough without dyspnea, and intermittent high-intensity pain in the left chest and spine. These manifestations led to the diagnosis of a left upper lobe mass (42×26 mm) that spread towards the mediastinum getting in contact with the left pulmonary artery and the lobar branch for the left upper lobe (Figure 1A). Initial images found lymph nodes in stations 2L and 5, several pulmonary nodules with a metastatic appearance (5 mm), and bone involvement at the level of the skull and right acetabulum (Figure 1B; November 2007). The tumor was then classified as stage IV (T2N1M1b according to the current TNM classification) and the pathology confirmed presence of a papillary adenocarcinoma positive for TTF1+/Ck7+ (Figure 1C). Thus, she was treated with a six-cycle platinum-based chemotherapy doublet (carboplatin/palitaxel), intervention that achieved a partial response that lasted 8 months. The tumor progressed in May 2008 at bone (Figure 2A,2B), and pemetrexed was then unsuccessfully administered for 3 months [maintaining a performance status (PS) score of 0]. In August 2008 the first analysis of EGFR mutations was done (using basal tissue and being one of the first patients in LATAM), demonstrating the presence of the mutation c.2236_2250del (p. Glu746_Ala750del) (Figure 2C). She started treatment with erlotinib plus ibandronic acid, achieving a partial response that lasted 42 months. At that time, focal pelvic progression was found (March 2012) and then the patient was treated with conformal radiation therapy. The first evaluation to detect the T790M resistance mutation in liquid biopsy (LB) was done by competitive allele-specific TaqMan PCR in 2012, study that was negative. Due to the presence of an oligoprogression, it was proposed to start erlotinib plus bevacizumab (15 mg/kg) intervention that achieved stable disease between June 2012 and October 2013. A PET/CT (November of 2013) confirmed extensive pelvic bone involvement, as well as in the dorsal and lumbosacral spine, and at the skull with slight functional decline (PS 1) (Figure 3A,3B). Given the suspicion of myeloptysis the bone marrow (BM) biopsy was positive. Then afatinib was successfully administered at 40 mgq/day for 1 year (stable disease). However, in December 2014 intensity-modulated radiation therapy (IMRT) was performed on the dorsal spine due to imaging signs of progression and increased pain. Then treatment was switched to a sixth-line prescription using afatinib (30 mg q/day) plus cetuximab. The disease remained stable for 14 months in a context of manageable grade 2 skin toxicity (during this period two additional LBs were negative for the T790M mutation). In February 2016, we found the T790M mutation; however, in the absence of osimertinib the treatment was rotated to afatinib plus weekly paclitaxel. This combination allowed a partial response with excellent physical condition (PS 0; she received 18 cycles between February 2016 and April 2017) and grade 2 neuropathy. The tumor progressed in the skull, the lateral region of the left orbit and in the primary tumor, findings for which cranial (segmental) IMRT and stereotactic body radiation therapy (SBRT) were performed on the orbital and pulmonary lesions (Figure 4A-4C). It was proposed that our patient join a trial testing the usefulness of irinotecan/bevacizumab combination in heavily treated lung adenocarcinoma patients. After finding low mRNA expression for TIMP1, she received this regimen for 11 months, achieving a partial response that was maintained until July 2018. The disease progressed in the pelvis, for which IMRT was performed. Interestingly, a new molecular analyzes by NGS in LB (Foundation One Liquid) revealed the persistence of the T790M mutation plus the appearance of TP53R213 mutation. Then osimertinib was successfully administered for 10 months. In August 2019, the patient presented bone, lung, and meningeal progression in relation to marked elevation of serum chromogranin (982 ng/dL) and clinical deterioration (PS 2; Figure 5A). Due to the suspicion of transdifferentiation to a SCLC with rapid bone progression a lung biopsy was performed by thoracoscopy. Histological analysis confirmed the presence of a SCLC (Figure 5B), and a new NGS test done in tumor tissue showed the persistence of EGFR and TP53R213 mutations and the appearance of the RB1Y709C. Carboplatin/etoposide/osimertinib was then unsuccessfully administered for 3 months with progressive neurological deterioration due to greater meningeal involvement (Figure 5C). The patient died in January 2020, after 12.5 years of OS and 10 lines of treatment. In Figure 6 is shown the case timeline, including diagnostic and therapeutic interventions.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family.
Discussion
Before introduction of targeted therapy, standard treatment for NSCLC, was platinum-doublet chemotherapy and outcomes were modest (23). With the advent of targeted therapy as treatment for LC patients, especially for EGFR-mutated NSCLC, important clinical benefits were found. Improvements in median PFS (approximately 9–18 months), longer OS to 21–45 months and changing treatment-related adverse events (AEs) compared to standard treatment options, are some of the advantages (6,22-24). As Yuan et al. reported, many clinical trials showed that patients with EGFR-mutant positive, have better clinical outcomes if they are treated with TKIs compared to standard chemotherapy (6). Therefore, at the moment of diagnosis, our patient started treatment with platinum-doublet chemotherapy, which was the best option at that time. After introduction of EGFR mutations analysis, and the finding of an exon 19 deletion, we started treatment with erlotinib. However, it is also known that after a median time of 12 months, resistance against TKI therapy developed inevitably and disease progression started (21). To overcome resistance and maximize OS our first attempt to reverse resistance involved the use of erlotinib in combination with bevacizumab. A recent meta-analysis assessed the effectiveness of erlotinib combined with bevacizumab (four studies) or ramucirumab (one study) (25). Compared to erlotinib monotherapy as first-line therapy, erlotinib plus antiangiogenic agents remarkably prolonged PFS (HR: 0.59, 95% CI: 0.51–0.69; P=0.000). However, overall response rate (ORR), disease control rate (DCR), and OS were similar between groups. Integration of information also found that overall grade 3–5 AEs increased in combination group (OR: 5.772, 95% CI: 2.38–13.94; P=0.000), particularly the incidence of diarrhea, acneiform rash, hypertension, and proteinuria. Additionally, subgroup analysis demonstrated that Asian patients could significantly benefit from combination therapy (HR: 0.59, 95% CI: 0.50–0.69; P=0.000), as well as patients with exon 19 deletions (HR: 0.61, 95% CI: 0.49–0.75; P=0.000) (25). The use of the erlotinib and bevacizumab combination has also shown an effect on leptomeningeal involvement (26). Recently, Grommes et al. reported the efficacy of high-dose erlotinib for brain and leptomeningeal metastases since drug concentrations in the cerebrospinal fluid (CSF) were shown to exceed the half-maximal inhibitory concentration for EGFR mutation-positive LC cells in a patient with neuroaxis involvement (27). Another previous study has suggested that CSF concentrations of vascular endothelial growth factor (VEGF) may be higher in patients with leptomeningeal disease and could correlate with a poor prognosis (28). This finding explains, at least in part, the efficacy of bevacizumab for CNS metastases and a recent study has demonstrated its efficacy and safety (29).
After erlotinib, and before the osimertinib era, our patient received afatinib in combination with cetuximab and then with paclitaxel. Preclinically, afatinib plus cetuximab overcomes T790M-mediated resistance. Previously, Janjigian et al. reported a phase Ib study combining afatinib and cetuximab in heavily pretreated EGFR-mutant patients with acquired resistance to erlotinib/gefitinib (30). Among 126 patients, ORR was 29% comparable in T790M-positive and T790M-negative tumors (32% vs. 25%; P=0.341). In addition, median PFS was 4.7 months, and the median duration of confirmed objective response was 5.7 months. Nevertheless, therapy-related grade 3/4 AEs occurred in 44%/2% of patients (30). In favor of this hypothesis, and especially in those with cerebral or leptomeningeal involvement, a preclinical study found that triplet therapy with afatinib, cetuximab, and bevacizumab induced pathological complete remission in xenograft tumors with H1975 and RPC-9 cells versus tumors treated with single or double therapies. This triple combo induced a significant reduction in CD31-positive vascular endothelial cells and increased cleaved caspase-3-positive cells in the tumors suggesting that one mechanism underlying deep remission, could be suppression of neovascularization and induction of apoptosis by intensive inhibition of driver oncoproteins and VEGF (31). The use of afatinib beyond progression and in combination with paclitaxel was based on the preliminary findings of the phase III randomized LUX-Lung 5 trial (32). Finally, the PFS (median 5.6 vs. 2.8 months, HR: 0.60; P=0.003) and ORR (32.1% vs. 13.2%; P=0.005) significantly improved with afatinib bases combination. Global health status/quality of life was maintained with afatinib plus paclitaxel over the entire treatment period.
Over the years, our patient developed resistance against first- and second-generation EGFR-TKIs and the disease progression was unavoidable. Meanwhile, we carried out three evaluations to detect the T790M resistance mutation, which is the most common and firstly reported mutation identified in the TK domain of already mutated EGFR (33). Finally, after 9 years and six lines of treatment we found this mutation. Girard et al. reported that some studies have identified T790M as a predominant resistance mechanism in patients treated with afatinib, the second-generation TKI that our patient was on at that moment (23). Before starting osimertinib (not available in the country at that time) our patient received the combination of irinotecan plus bevacizumab with success. A meta-analytic review involving 1,473 patients with previously untreated stage IIIB/IV NSCLC, demonstrated that irinotecan-based and non-based chemotherapy were associated with similar ORR (RR: 1.08, 95% CI: 0.94–1.23; P=0.30), OS (HR: 0.97, 95% CI: 0.88–1.07; P=0.56), and PFS (HR: 1.02, 95% CI: 0.97–1.08; P=0.38) (34). However, subgroups between Asian and non-Asian patients differed significantly in OS, finding reproducible in Hispanics considering the population admixture. Previously, our group showed benefit of the combination of irinotecan plus bevacizumab in patients with EGFR mutations, especially in the presence of low levels mRNA TIMP1 expression (a condition that our patient had) (35). In the same way, Pesta et al. reported a strong relationship between high levels of TIMP1 mRNA expression and adverse prognosis (36).
Based on the results of the AURA studies (18), our patient finally received osimertinib after revealing persistence of the T790M mutation plus the appearance of TP53R213 mutation. TP53 is a gene that codes for a tumor suppressor protein (33). Piper-Vallillo et al. and Liu et al., reported that finding a non-disruptive TP53 mutation in EGFR-mutant NSCLC increased the risk of transformation to SCLC during disease course (12,21). It is known, that osimertinib resitance could be developed by multiple mechanisms and histologic transformation is one of them. SCLC transformation is strongly associated with loss of RB1 (retinoblastoma protein) and TP53, and occurs in approximately 3–5% of patients whose cancer progresses during treatment with EGFR-TKIs (12,21). Histologic transformation mechanism is not fully understood, but as Liu et al. reported, it is believed that alveolar type II cells may be common precursors of both types of LC, and because of the EGFR mutations the cells might trans-differentiate to SCLC under the selective pressure of TKI therapy (21). As we described, after 10 months of treatment with third-generation TKI, disease progressed and transdifferentiation to SCLC was suspected. It is important to highlight, that plasma testing cannot be used to screen for transformation, and tissue biopsies must be performed to confirm the diagnosis (12). Therefore, histological analysis was conducted and confirmed the transformation. Additionally, NGS test done in tumor tissue demonstrated the persistence of the TP53R213 mutation and the appearance of the RB1Y709C, both strongly associated with histologic variation (12,21).
Sadly, after administration of first and second EGFR-TKI generations, our patient developed meningeal compromise, finding that spread rapidly after treatment with osimertinib started, and she died less than a year after. On FLAURA trial was reported that, osimertinib extends PFS in untreated EGFR-mutated advanced NSCLC patients with BM versus first-generation EGFR-TKI (15.2 vs. 9.6 months; P<0.001) (9,12). It is known that, osimetrinib penetrates easily the CNS and that has likely contributed to the good response (12). Recently, Park et al. informed the results of a phase II trial designed to test the role of osimertinib in patients with leptomeningeal disease after exposure to another TKI. The study found in the leptomeningeal cohort an intracranial DCR of 92.5% and a complete RR of 12.5%. The median OS was 13.3 months (95% CI: 9.1–NR) and the PFS was 8.0 months (95% CI: 7.2–NR). Subgroup analyses based on previous exposure to T790M-targeting agents, including osimertinib 80 mg or other third-generation EGFR-TKIs, showed no difference in PFS in cases with meningeal involvement (37).
Our patient died in January 2020, after 12.5 years of OS and 10 lines of medical treatment. Despite prognosis, some studies reported long time survival in patients with EGFR-mutant NSCLC treated with target therapy. In 2015 Kempf et al., reported a 10-year survival from a patient with metastatic EGFR-mutated NSCLC that went through multiple medical and surgical treatments (38). Later, in 2016 Lin et al., reported a 5-year survival of 14.6% in a cohort of 137 patients treated with TIKs form first and second generation (7). Afterwards, in 2018 Huang et al., reported a 5-year survival of 5% among a cohort of 1030 patients, they concluded that absence of extrathoracic spread and EGFR-TKI treatment of more than a year play an important role and are associated with long term survival (22). To our knowledge, this article presents one of the longest reported survivals of a metastatic LC patient with EGFR mutation.
To conclude with the approval of new generations of EGFR-TKIs, the challenge that we facing is the identification of optimal strategies to treat individual patients, with the aim of maximizing OS avoiding drug resistance. As we reported, we pursued to offer our patient the best personalized treatment at the time, with the aim of maximizing her OS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-53
Peer Review File: Available at http://dx.doi.org/10.21037/pcm-20-53
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-53). OA reports personal fees from Pfizer, grants and personal fees from Astra Zeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Lilly, personal fees from Merck, personal fees from Bristol Myers Squibb, grants and personal fees from Roche, outside the submitted work. GR reports personal fees from ROCHE, AMGEN, and PFIZER, as well as grants from AMGEN, outside the submitted work. AFC discloses financial research support from Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb and The Foundation for Clinical and Applied Cancer Research-FICMAC. Additionally, he was linked and received honoraria as advisor, participate in speakers’ bureau and gave expert testimony to Merck Sharp & Dohme, Boehringer Ingelheim, Roche, Bristol-Myers Squibb, Pfizer, Novartis, Celldex Therapeutics, Foundation Medicine, Eli Lilly and Foundation for Clinical and Applied Cancer Research-FICMAC. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s family.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gómez XE, Soto A, Gómez MA. Survival and prognostic factors in non-small cell lung cancer patients with mutation of the EGFR gene treated with tyrosine kinase inhibitors in a peruvian hospital. Am J Cancer Res 2019;9:1009-16. [PubMed]
- Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health 2019;85:8. [Crossref] [PubMed]
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer. (cited 2020 Sep 10). Available online: https://gco.iarc.fr/today
- Merchant N, Nagaraju GP, Rajitha B, et al. Matrix metalloproteinases: their functional role in lung cancer. Carcinogenesis 2017;38:766-80. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Yuan M, Huang LL, Chen JH, et al. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct Target Ther 2019;4:61. [Crossref] [PubMed]
- Lin JJ, Cardarella S, Lydon CA, et al. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thorac Oncol 2016;11:556-65. [Crossref] [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Chen YH, Chen YF, Chen CY, et al. Clinical factors associated with treatment outcomes in EGFR mutant non-small cell lung cancer patients with brain metastases: a case-control observational study. BMC Cancer 2019;19:1006. [Crossref] [PubMed]
- Abdallah SM, Wong A. Brain metastases in non-small-cell lung cancer: are tyrosine kinase inhibitors and checkpoint inhibitors now viable options? Curr Oncol 2018;25:S103-14. [Crossref] [PubMed]
- Lee JS, Hong JH, Sun DS, et al. The impact of systemic treatment on brain metastasis in patients with non-small-cell lung cancer: a retrospective nationwide population-based cohort study. Sci Rep 2019;9:18689. [Crossref] [PubMed]
- Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J Clin Oncol 2020; Epub ahead of print. [Crossref] [PubMed]
- Arrieta O, Cardona AF, Federico Bramuglia G, et al. Genotyping non-small cell lung cancer (NSCLC) in Latin America. J Thorac Oncol 2011;6:1955-9. [Crossref] [PubMed]
- Carrot-Zhang J, Soca-Chafre G, Patterson N, et al. Genetic ancestry contributes to somatic mutations in lung cancers from admixed Latin American populations. Cancer Discov 2021;11:591-8. [Crossref] [PubMed]
- Shah R, Lester JF. Tyrosine kinase inhibitors for the treatment of EGFR mutation-positive non-small-cell lung cancer: a clash of the generations. Clin Lung Cancer 2020;21:e216-28. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]
- Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 2020;26:2654-63. [Crossref] [PubMed]
- Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: a case report and literatures review. Cancer Biol Ther 2018;19:445-9. [Crossref] [PubMed]
- Huang CY, Chen BH, Chou WC, et al. Factors associated with the prognosis and long-term survival of patients with metastatic lung adenocarcinoma: a retrospective analysis. J Thorac Dis 2018;10:2070-8. [Crossref] [PubMed]
- Girard N. Optimizing outcomes and treatment sequences in EGFR mutation-positive non-small-cell lung cancer: recent updates. Future Oncol 2019;15:2983-97. [Crossref] [PubMed]
- Noronha V, Patil VM, Joshi A, et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol 2020;38:124-36. [Crossref] [PubMed]
- Chen F, Chen N, Yu Y, et al. Efficacy and safety of epidermal growth factor receptor (EGFR) inhibitors plus antiangiogenic agents as first-line treatments for patients with advanced EGFR-mutated non-small cell lung cancer: a meta-analysis. Front Oncol 2020;10:904. [Crossref] [PubMed]
- Sakata Y, Kawamura K, Shingu N, et al. Erlotinib plus bevacizumab as an effective treatment for leptomeningeal metastases from EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2016;99:120-2. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro-Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Herrlinger U, Wiendl H, Renninger M, et al. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer 2004;91:219-24. [Crossref] [PubMed]
- Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, untreated brain metastases (BRAIN): a nonrandomized, phase II study. Clin Cancer Res 2015;21:1896-903. [Crossref] [PubMed]
- Janjigian YY, Smit EF, Groen HJM, et al. Dual inhibition of EGFR with afatinib and cetuximab in kinase inhibitor-resistant EGFR-mutant lung cancer with and without T790M mutations. Cancer Discov 2014;4:1036-45. [Crossref] [PubMed]
- Kudo K, Ohashi K, Makimoto G, et al. Triplet therapy with afatinib, cetuximab, and bevacizumab induces deep remission in lung cancer cells harboring EGFR T790M in vivo. Mol Oncol 2017;11:670-81. [Crossref] [PubMed]
- Schuler M, Yang JCH, Park K, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: phase III randomized LUX-Lung 5 trial. Ann Oncol 2016;27:417-23. [Crossref] [PubMed]
- Kibirova A, Mattes MD, Smolkin M, et al. The Journey of an EGFR-Mutant Lung Adenocarcinoma through Erlotinib, Osimertinib and ABCP Immunotherapy Regimens: Sensitivity and Resistance. Case Rep Oncol 2019;12:765-76. [Crossref] [PubMed]
- Yang XQ, Li CY, Xu MF, et al. Comparison of first-line chemotherapy based on irinotecan or other drugs to treat non-small cell lung cancer in stage IIIB/IV: a systematic review and meta-analysis. BMC Cancer 2015;15:949. [Crossref] [PubMed]
- Wills B, Cardona AF, Rojas L, et al. Survival outcomes according to TIMP1 and EGFR expression in heavily treated patients with advanced non-small cell lung cancer who received biweekly irinotecan plus bevacizumab. Anticancer Res 2017;37:6429-36. [PubMed]
- Pesta M, Kulda V, Kucera R, et al. Prognostic significance of TIMP-1 in non-small cell lung cancer. Anticancer Res 2011;31:4031-8. [PubMed]
- Park S, Lee MH, Seong M, et al. A phase II, multicenter, two cohort study of 160 mg osimertinib in EGFR T790M-positive non-small-cell lung cancer patients with brain metastases or leptomeningeal disease who progressed on prior EGFR TKI therapy. Ann Oncol 2020;31:1397-404. [Crossref] [PubMed]
- Kempf E, Planchard D, Le Chevalier T, et al. 10-year long-term survival of a metastatic EGFR -mutated nonsmall cell lung cancer patient. Eur Respir J 2015;46:280-2. [Crossref] [PubMed]
Cite this article as: Ordóñez-Reyes C, Ruiz-Patiño A, Arrieta O, Zatarain-Barrón L, Rojas L, Recondo G, Ricaurte L, Cardona AF; Latin American Consortium for the Investigation of Lung Cancer (CLICaP). Precision oncology in EGFR positive non-small cell lung cancer: breaking the 10-year barrier—a case report. Precis Cancer Med 2021;4:5.

