
Feasibility of ultra-central stereotactic ablative irradiation in lung cancer undergoing nivolumab: a case report
Introduction
The role of radiotherapy in oligometastatic lung cancer patients has proven to be able to achieve long-term progression-free survival and its synergy with the novel molecules is a promising field of investigation (1).
Central localizations, defined as tumor located within 2 cm of the proximal bronchial tree, great vessels, trachea, heart, or other mediastinal structures, may be correlated to a higher incidence of severe adverse events (2). A subset of proximally central tumors called “ultra-central” tumors has recently been identified, and is characterized by the overlapping of the planned target volume (PTV) with the proximal bronchial tree, trachea, esophagus, pulmonary vein or pulmonary artery (3). For this subset of localizations, the optimal dose and fractionation, as well as the safety are still to be determined (4). At the same time, the synergy with chemotherapy or immunotherapy is still unknown.
Aim of this report is the description of a metastatic non-small cell lung cancer (NSCLC) patient undergoing nivolumab successfully treated with stereotactic radiation therapy in an ultracentral mediastinal metastasis.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-40).
Case presentation
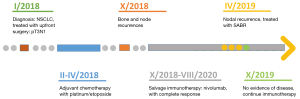
The reported patient was a 45-year-old male that was diagnosed in January 2018 with a locally advanced lung adenocarcinoma. The patient underwent upfront surgery (pT3N1) and adjuvant therapy (four cycles of chemotherapy with platinum 75 mg/m2 and etoposide 100 mg/m2, every 3 weeks).
The subsequent follow-up examinations were negative till October 2018, when a restaging computed tomography (CT) showed bone and node recurrence. The patient started immunotherapy with Nivolumab 40 mg/m2 every 2 weeks, and achieved a complete response of disease.
Subsequent restaging was negative till April 2019, when a positron emission tomography (PET)/CT showed an adenopathy in the subcarinal area characterized by an increase in FDG consumption (SUVmax 5.5) (see Figure 1). The patient was still undergoing Nivolumab, 40 mg/m2 every 2 weeks, and was referred to our Unit.
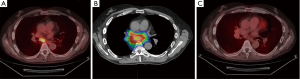
The patient underwent 4D simulation CT scan and we performed an elastic fusion between simulation CT and both diagnostic CT and PET/CT scans. The gross target volume (GTV) was delineated on diagnostic scans and transferred to simulation CT. The GTV was deformably propagated across phases to obtain internal target volume (ITV) using MIM Maestro software, a commercially deformable image registration tool (MIM Software Inc., Cleveland, OH, USA). The quality of contour propagation was inspected visually by a clinician with 25 years of experience in lung radiotherapy, and modifications were made where required. ITV was then expanded to 5 mm to obtain PTV. The radiation dose was chosen taking into consideration the dose constraints of the closer organs at risk (esophagus, trachea, aorta, heart).
The patient underwent stereotactic radiation therapy on the target lymph node, with 44 Gy in eight fractions (5.5 Gy/day, daily), prescribed at the isodose of 80% of the PTV (see Figure 1).
The treatment started during Nivolumab treatment, and subsequent immunotherapy cycle were not deferred.
The patient reported no acute or sub-acute toxicity, during the radiation treatment and in the following weeks. A CT scan performed 2 months after SABR showed no signs of toxicity and a partial response of the nodal recurrence.
The patient is still undergoing immunotherapy and last CT examination, in June 2020, show no signs of recurrence of disease.
The PET/CT scan performed 6 months after the end of the radiotherapy showed a complete metabolic response of the irradiated lesion, with no signs of recurrence of disease in other districts (see Figures 1,2).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
Metastatic lung cancer has been synonymous with a lethal outcome and is generally considered incurable, although during the last decades there has been a slight improvement in overall survival (OS). Survival rates, however, standardized for age shows very little variations among 67 countries (10–20%), and this may in part reflect generally unsuccessful therapeutic strategies (5).
It is of great interest to define subgroups of patients suffering from this heterogeneous disease that might benefit from different treatment strategies. One special subgroup comprises patients with limited tumour spread lying between localized cancers and disseminated metastatic cancer, termed oligometastatic disease. In particular, oligometastatic tumours are characterized by low volume metastatic disease with limited number and size of metastatic lesions (up to five and not necessarily in the same organ), potentially amenable for local treatment, aimed at achieving long-term remission (6).
At this regard, the role of radiation therapy especially for oligometastatic lung cancer patients, has shown to be able to achieve long-term progression-free survival, without significant treatment related toxicities, as in other pathologies (7). Its investigation with the novel molecules will be necessary in the next future for the optimal management of lung cancer patients, elderly included.
The association between immunotherapy and radiotherapy is currently under investigation in clinical trials, as it is considered a potential weapon in patients with metastatic NSCLC, especially in the oligometastatic setting.
Ultracentral localizations of NSCLC, at the same time, represent serious concerns regarding the balance of toxicities and outcomes (4,8), as this region was historically considered a “no fly zone” (9). Several studies have tried to develop different stereotactic approach for central tumors (10-12), although scarce data are available for ultra-central tumors (4). Other strategies that are under investigation include proton therapy or hadrontherapy (13).
Loi et al. showed that a biologically effective dose (BED) >75 Gy correlated with a significant gain in both progression-free survival and OS (4). Our patient underwent a BED of 68.2 Gy due to surrounding organs at risks. In literature median radiotherapy related grade >2 toxicities were 10% and treatment related mortality rate was 5% (14).
Systemic therapies are often correlated with poorer outcomes, as chemotherapy-naïve patients are frequently more radiosensitive in comparison to heavily-pretreated patients (15). Immunotherapy, at our knowledge, is still not investigated in the context of ultracentral radiotherapy.
In our case description we have shown a successful treatment of an oligometastatic NSCLC patient that underwent radiotherapy to ultracentral localization concomitant with immunotherapy treatment.
The strength of the reported case is the successful radiotherapy treatment of a critical localization, whereas the limitations are the impossibility of achieve a BED >100 Gy.
The association between immunotherapy and radiation seems to be useful in terms of outcomes, although at the present time we need more data on the safety of this combined approach in the subset of ultra-central localizations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-40
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-40). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Giuliani M, Mathew AS, Bahig H, et al. SUNSET: Stereotactic Radiation for Ultracentral Non-Small-Cell Lung Cancer-A Safety and Efficacy Trial. Clin Lung Cancer 2018;19:e529-32. [Crossref] [PubMed]
- Loi M, Franceschini D, Dominici L, et al. Stereotactic Radiotherapy for Ultra-Central Lung Oligometastases in Non-Small-Cell Lung Cancer. Cancers (Basel) 2020;12:885. [Crossref] [PubMed]
- Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015;385:977-1010. Erratum in: Lancet 2015 Mar 14;385(9972):946. [Crossref] [PubMed]
- Palacios-Eito A, García-Cabezas S. Oligometastatic disease, the curative challenge in radiation oncology. World J Clin Oncol 2015;6:30-4. [Crossref] [PubMed]
- Trovo M, Furlan C, Polesel J, et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother Oncol 2018;126:177-80. [Crossref] [PubMed]
- Jereczek-Fossa BA, Muto M, Durante S, et al. Stereotactic body radiation therapy for mediastinal lymph node metastases: how do we fly in a 'no-fly zone'? Acta Oncol 2018;57:1532-9. [Crossref] [PubMed]
- Senan S. Stereotactic body radiotherapy: do central lung tumors still represent a 'no-fly zone'? Onkologie 2012;35:406-7. [Crossref] [PubMed]
- Chang JY, Li QQ, Xu QY, et al. Stereotactic ablative radiation therapy for centrally located early stage or isolated parenchymal recurrences of non-small cell lung cancer: how to fly in a "no fly zone". Int J Radiat Oncol Biol Phys 2014;88:1120-8. [Crossref] [PubMed]
- Mangona VS, Aneese AM, Marina O, et al. Toxicity after central versus peripheral lung stereotactic body radiation therapy: a propensity score matched-pair analysis. Int J Radiat Oncol Biol Phys 2015;91:124-32. [Crossref] [PubMed]
- Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014;90:1168-76. [Crossref] [PubMed]
- Chi A, Chen H, Wen S, et al. Comparison of particle beam therapy and stereotactic body radiotherapy for early stage non-small cell lung cancer: A systematic review and hypothesis-generating meta-analysis. Radiother Oncol 2017;123:346-54. [Crossref] [PubMed]
- Chen H, Laba JM, Zayed S, et al. Safety and Effectiveness of Stereotactic Ablative Radiotherapy for Ultra-Central Lung Lesions: A Systematic Review. J Thorac Oncol 2019;14:1332-42. [Crossref] [PubMed]
- Franceschini D, De Rose F, Franzese C, et al. Predictive Factors for Response and Survival in a Cohort of Oligometastatic Patients Treated With Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys 2019;104:111-21. [Crossref] [PubMed]
Cite this article as: Nardone V, Calvanese MG, Mormile M, Giugliano FM, Correale P, Reginelli A, Cappabianca S, Guida C. Feasibility of ultra-central stereotactic ablative irradiation in lung cancer undergoing nivolumab: a case report. Precis Cancer Med 2020;3:28.

