
Central nervous system progression and liquid biopsy in patients with oncogene addicted non-small cell lung cancer treated with ALK/ROS1 inhibitors
Introduction
Oncogene-addicted non-small cell lung cancer (NSCLC) patients frequently develop central nervous system (CNS) metastases, with percentages reaching up to 50% for brain and 10% for leptomeningeal metastases (1-3). ALK- and ROS1-positive tumors have a high CNS tropism, with an incidence of CNS metastases at baseline of 36% and 34%, respectively (4), a risk of CNS progression of 58% at 3 years (5) and up to 70% at progression during crizotinib treatment (1,6,7). Also, in a significant proportion of cases, patients develop isolated CNS metastases, especially when treated with first-generation tyrosine kinase inhibitors (TKIs), due to an insufficient drug penetration of the blood-brain barrier (4,8,9).
CNS metastases are associated with a dismal prognosis and impact the quality of life of cancer patients, which highlights the importance of administering an effective treatment for CNS disease. Novel generation TKIs, including second (alectinib, ceritinib, brigatinib) and third generation (lorlatinib) ALK and ROS1 inhibitors proved an increased intracranial activity with long duration of responses (10).
The use of TKIs frequently result in the development of on-target or by-pass resistance alterations. In ALK-rearranged NSCLC, ALK mutations occur in 30% of patients under crizotinib, in up to 54–70% after second generation ALK inhibitors and may accumulate with the sequential use of TKIs (11,12). There is a wide spectrum of resistance alterations that have their own sensitivity and need drugs with different structures, that do not have the same resistance profile (13). Similarly, ROS1-rearranged NSCLC may develop various resistance mutations under crizotinib, which may have different sensitivity profiles for newer generation inhibitors (14). Thus, the identification of resistance alterations might better guide treatment choice and tailor treatment according to the “sensitivity profile” of each alteration. This might increase chances of using the most adequate treatment, which is essential for patients with CNS progression, at risk of rapid neurological deterioration.
However, obtaining a molecular profile of the CNS progression is challenged by the invasive nature of tissue biopsies, which are not feasible in the majority of cases. An alternative technique is the analysis of circulating tumor DNA (ctDNA), which is shed by tumors in the blood or other liquids, such as the cerebro-spinal fluid (CSF), pleural or peritoneal collections. ctDNA analysis, named “liquid biopsy” is a minimally invasive technique, that may detect genomic alterations and resistance mutations with high sensitivity and specificity (15,16).
Here, we discuss the interest and feasibility of liquid biopsy in patients with ALK- or ROS1-rearranged tumors with CNS metastases.
TKIs and CNS metastases
In ALK-rearranged NSCLC, crizotinib, the first approved ALK inhibitor, has superior intracranial disease control rates compared to standard chemotherapy, but the insufficient CNS penetration of crizotinib leads to that CNS is the main site of disease progression (7,17). Next-generation ALK inhibitors have superior intracranial activity compared to crizotinib and/or chemotherapy, with early and durable CNS responses (18-26). Importantly, second generation ALK inhibitors have also an intra-cranial activity after crizotinib failure, while lorlatinib, a third generation TKI inhibitor, is active also in heavily pre-treated patients who fail at least two lines of therapy (27). Lorlatinib is very active in patients with CNS disease because of its high CNS penetration that reach 75% of plasma levels in the CSF, and because it is able to treat the majority of ALK resistance mutations (27,28).
In ROS1-rearranged NSCLC, ROS1/ALK inhibitors are highly active, including in patients with brain metastases. Crizotinib, the first approved ROS1 inhibitor, showed an objective response rate of 71.7%, regardless of the presence of brain metastases at baseline. In 23 patients with measurable brain metastases at baseline, the median progression-free survival (PFS) was 10.2 months (29). In crizotinib-naïve patients with baseline brain metastases, next generation ROS1 inhibitors have increased intracranial activity, as reported with ceritinib in 5 out of 8 patients in a phase II study (30) and entrectinib, an ALK/ROS1/pan-TRK inhibitor, in 55% of patients in a pooled analysis of phase I and II studies (31). Repotrectinib, another next generation ROS1/TRK/ALK inhibitor, was shown to have intracranial activity in in vivo and clinical studies (32,33). In crizotinib-pretreated NSCLC patients with baseline CNS metastases included in the IFCT-1803 LORLATU expanded access program cohort, lorlatinib showed intracranial ORR of 37.7%, similar to ALK-positive patients (34).
Tailoring treatment based on the molecular profile might improve patients’ outcomes
As next generation ALK and ROS1 inhibitors have different “sensitivity” profiles (10,14,32,35,36) (Figure 1), a tailored treatment based on the identified resistance mutations might gain additional lines of therapy, while also increasing the chance of obtaining a CNS response. In case of ALK-positive tumors, lorlatinib may be effective in the majority of cases, especially when the ALK G1202 mutation is found, which is resistant to all other ALK inhibitors and it is the main resistance mutation after the current standard of care, second generation TKI (13). Promising preclinical data presented at AACR 2020 point out towards TPX-031, a novel ALK inhibitor, with high potency against the wild-type ALK and hard to treat mutations, such as the solvent front mutation G1202R and the compound mutation G1202R/L1196M. In case of ROS1 positive tumors, the identification of ROS1 G2032R could directly tailor patients for the use of repotrectinib and not lorlatinib, which will not be active in this situation (32). The identification of compound mutations that drive resistance to third generation inhibitors, might be overcome in selected cases by first or second-generation inhibitors with different molecular structures or they might even resensitize tumors to first generation inhibitors (37,38). Also, emergent by-pass alterations might benefit from personalized drug combinations, such as dual ALK/MET blockade in tumors acquiring MET alterations (39).
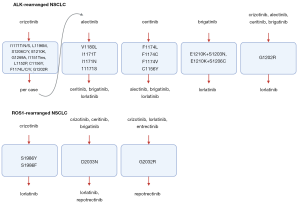
Clinical trials evaluating drugs matched to the identified resistance alterations will help clarify the clinical utility of a personalized approach. For instance, the ongoing National Cancer Institute (NCI)-NRG ALK Protocol phase II randomized trial (NCT03737994) investigates the use of tumor and liquid biopsies in 660 ALK-positive NSCLC patients failing a next-generation inhibitor, who receive a matched treatment according to the identified resistance mutation. The ALKALINE (NCT04127110) and the ORAKLE (NCT04111705) phase II studies aim to assess the clinical activity of lorlatinib in NSCLC patients failing second generation inhibitors, based on the molecular pattern of resistance to prior ALK inhibitors, assessed by liquid biopsy and tissue or liquid biopsy, respectively.
Clinical relevance of liquid biopsy in the characterization of CNS lesions
As performing CNS tissue biopsies is highly invasive for the patient, liquid biopsy by ctDNA appears as an easy, minimally invasive technique, able to rapidly provide a tumor molecular profiling (15).
In ALK-positive NSCLC, plasma liquid biopsy has been proven to be clinically relevant at detecting the fusion and resistance mutations after failing TKIs. In 101 NSCLC patients positive for the ALK fusion, targeted next-generation, amplicon-based sequencing has shown a sensitivity of 67% for the fusion detection at baseline and 46% at TKI failure. Resistance mutations following TKIs were detected in 22% of ALK-positive cases at progression to first and next generation ALK TKIs (29% after second generation ALK TKI), with ALK G1202R the most frequently detected. Interestingly, the authors studied the efficacy of the next therapy based on the presence of molecular alterations in blood. The presence of resistance alterations was associated with an overall survival of 58.5 months in case of ALK mutations and 44.1 months in case of other alterations. A negative liquid biopsy (no alteration detected) at TKI failure was associated with improved outcomes (105 months), that may be related to the low tumor burden of these patients. Among patients with ALK mutations, patients harboring complex ALK-resistance mutations had the poorest outcomes to next therapy (40). In another study on 76 ALK-positive NSCLC, a high concordance of the ALK fusion was obtained between plasma and tissue in 91% of cases (20/22 blood/tissue samples). In 15 patients failing a TKI, 24 ALK resistance mutations were detected, the most commonly observed being ALK G1269A (4/24 alterations) (41).
In 27 ROS1-positive NSCLC, plasma liquid biopsy by amplicon-based sequencing identified resistance mutations following TKIs in 30% of ROS1-positive cases, with ROS1 G2032R being the most frequently detected. Similar to ALK, a negative liquid biopsy at TKI failure was associated with improved outcomes, and the presence of G2032R at progression to crizotinib was associated with rapid progression to subsequent therapy, including lorlatinib (40). Similar results were obtained in 56 ROS1-positive NSCLC, where the detection of the ROS1 fusion at relapse following ROS1 inhibitors was 50% by plasma liquid biopsy. Six out of 18 patients failing crizotinib (33%) had ROS1 resistance mutations, the most frequently detected being the ROS1 G2032R mutation, in 5 out of 6 cases (42).
However, plasma liquid biopsy is unlikely to adequately characterize the molecular landscape of CNS metastases, as the blood-brain barrier could reduce the presence of ctDNA in the bloodstream. Our group has evaluated the clinical relevance of plasma liquid biopsy in oncogene-addicted NSCLC with isolated CNS progression, in comparison with patients with systemic progression. Although we used a highly sensitive next-generation sequencing assay, there was a high percentage of negative liquid biopsies in patients with isolated CNS progression (48%), as opposed to patients with systemic progression (8–16%). In several patients with longitudinal biopsies, a negative liquid biopsy at the moment of isolated CNS progression shifted to positive when there was a systemic progression. Moreover, patients with isolated CNS progression and positive liquid biopsies had a higher chance of developing earlier a subsequent extra-CNS progression, suggesting that plasma ctDNA was more likely to be shed by infra-radiologic, active extra-CNS lesions (9).
A more suitable way for characterizing the mutational landscape of CNS lesions is the CSF (43). As compared to plasma ctDNA, several studies have shown that CSF ctDNA had a higher mutation detection rates and higher allele frequencies of identified genomic alterations in a cohort of 72 NSCLC patients (44), in 26 EGFR driven-NSCLC (45) and 11 ALK rearranged NSCLC patients with leptomeningeal metastases (3). In the 11 paired plasma-CSF samples from ALK rearranged NSCLC patients, the driver alterations were detected in 9 out of 11 CSF samples (81.8%) and in 5 out of 11 plasma samples (45.5%). In one patient, two ALK resistance mutations were detected in CSF and not in plasma (3). Moreover, a significant number of unique mutations was found in CSF, such as copy number variants of EGFR, CCND1, FGF3 and FGF4 (3). Similarly, in a cohort of EGFR-driven NSCLC with leptomeningeal disease, acquired resistance alterations, such as EGFR T790M, copy-number variants of MET, ERBB2, and KRAS, and TP53 loss of heterozygosity, were detected in CSF ctDNA compared to plasma ctDNA, which mainly detected the initial EGFR-activating mutations (45). This could be explained by the fact that alterations with low allele frequencies might be easier detected in CSF where the low levels of non-tumor derived DNA lead to a high ctDNA/circulating free DNA ratio (46). Also, differences in tumor genomics could occur between the CNS and extra-CNS lesions because of reduced CNS drug penetration which could determine a different tumor selection in the CNS (47). This might explain the variable prevalence of EGFR T790M which has been reported within studies. In the study of Li et al., EGFR T790M was detected in 30.4% in CSF versus 21.7% in plasma after TKI progression at leptomeningeal metastasis diagnosis (45), while Ying et al. reported a higher EGFR T790M mutation detection rate in plasma than in CSF (15.3% plasma vs. 2.8% CSF, P=0.017) in 72 NSCLC patients with leptomeningeal metastases (44). In another study, EGFR T790M was rarely found in CSF at diagnosis of leptomeningeal metastases in patients exposed to TKI, in more cases under erlotinib than under gefitinib, suggesting that the emergence of resistance mutation might be correlated with the CSF concentration of first generation TKI (48).
While the relevance of CSF liquid biopsy is supported by several studies in patients with leptomeningeal metastases, less is known about its utility for patients with brain metastases. In 66 treatment naive NSCLC patients, Li et al. recently investigated the phenotypical features of brain metastases to predict the probability of detecting ctDNA in CSF. After considering tumor size and distance to ventricles, authors established a prediction model to select patients for CSF analysis with AUC 0.82, sensitivity of 90.6% and specificity of 73.9% (49). This work aimed to avoid invasive procedures (lumbar punctures) in patients with low chances of having a positive liquid biopsy. However, in addition to the amount of ctDNA that is shed in the CSF, the sensitivity of the liquid biopsy technique would also have an important contribution.
In addition to the molecular profile, another factor that should be considered at progression is the TKI concentration. In an exploratory cohort of 41 patients with oncogene-addicted NSCLC patients treated with TKI, the plasmatic TKI concentration at progression was lower than normal in 57% (n=21/37 samples) of cases, including 3 out of 4 patients with isolated CNS progression (50).
Unanswered questions and future perspectives
Could plasma liquid biopsy still be useful in case of isolated CNS progression?
The absence of any identified genomic alteration in a plasma liquid biopsy in an oncogene-addicted NSCLC patient with isolated CNS progression should be interpreted as a false negative result, most probably caused by an insufficient plasmatic ctDNA. In the absence of known drivers, aberrant ctDNA methylation may help to accurately identify and quantify ctDNA with respect to normal circulating free DNA (51). When the plasma liquid biopsy is found to be positive and genomic alterations are identified, it may reveal the molecular profile corresponding to the CNS progression but it may also predict for a subsequent extra-CNS progression (9). It would be interesting to evaluate this observation in prospective studies, as it may prove to be useful in the selection of CNS progressing patients for brain irradiation or for the switch of systemic therapy.
Could CSF liquid biopsy evaluate response to treatment in case of leptomeningeal disease?
The evaluation of treatment response in case of leptomeningeal meningitis is evaluated by radiological findings, CSF cytology and clinical evolution of neurological signs and symptoms (52). However, this is less precise than in case of brain metastases and often difficult to perform, especially in the absence of typical radiological findings. It may be interesting to investigate CSF liquid biopsy as a potential tool to evaluate response to treatment or even to guide subsequent therapeutic strategies in case of isolated leptomeningeal progression.
Conclusions
CNS progression is a common event in patients with ALK or ROS1 positive NSCLC. The molecular characterization of CNS disease can be performed by liquid biopsy, the most accurate being the CSF analysis. Tailoring treatment based on the molecular profile may improve patients’ outcomes and possibly lower the risk of rapid CNS deterioration as compared to a “blinded” treatment switch, currently under evaluation in prospective studies.
Acknowledgments
Figure created with BioRender.com.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Precision Cancer Medicine for the series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-41). The series “ALK and ROS-1 NSCLC Patients Treatment Approach Based on Genomic Profile by Liquid Biopsy” was commissioned by the editorial office without any funding or sponsorship. LM served as the unpaid Guest Editor of the series and. serves as an unpaid editorial board member of Precision Cancer Medicine from March 2020 to February 2022. CT reports grants and personal fees from Pfizer and Novartis, personal fees from Takeda, MSD, BMS, Roche, Diaceutics, outside the submitted work. Dr. LM reports personal fees from Roche Diagnostics, Takeda, Roche, personal fees from Bristol-Myers Squibb, personal fees from Tecnofarma, personal fees from Roche, personal fees from AstraZeneca, other from Chugai, Bristol-Myers Squibb, Roche, other from Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Remon J, Besse B. Brain Metastases in Oncogene-Addicted Non-Small Cell Lung Cancer Patients: Incidence and Treatment. Front Oncol 2018;8:88. [Crossref] [PubMed]
- Remon J, Le Rhun E, Besse B. Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 2017;53:128-37. [Crossref] [PubMed]
- Zheng MM, Li YS, Jiang BY, et al. Clinical Utility of Cerebrospinal Fluid Cell-Free DNA as Liquid Biopsy for Leptomeningeal Metastases in ALK-Rearranged NSCLC. J Thorac Oncol 2019;14:924-32. [Crossref] [PubMed]
- Patil T, Smith DE, Bunn PA, et al. The Incidence of Brain Metastases in Stage IV ROS1-Rearranged Non-Small Cell Lung Cancer and Rate of Central Nervous System Progression on Crizotinib. J Thorac Oncol 2018;13:1717-26. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 2010;116:1336-43. [Crossref] [PubMed]
- Aldea M, Hendriks L, Mezquita L, et al. Circulating Tumor DNA Analysis for Patients with Oncogene-Addicted NSCLC With Isolated Central Nervous System Progression. J Thorac Oncol 2020;15:383-91. [Crossref] [PubMed]
- Recondo G, Facchinetti F, Olaussen KA, et al. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol 2018;15:694-708. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK Inhibitors Can Select for Lorlatinib-Resistant Compound ALK Mutations in ALK-Positive Lung Cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Facchinetti F, Loriot Y, Kuo MS, et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin Cancer Res 2016;22:5983-91. [Crossref] [PubMed]
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J Thorac Oncol 2018;13:1248-68. [Crossref] [PubMed]
- Remon J, Lacroix L, Jovelet C, et al. Real-World Utility of an Amplicon-Based Next-Generation Sequencing Liquid Biopsy for Broad Molecular Profiling in Patients With Advanced Non-Small-Cell Lung Cancer. JCO Precis Oncol 2019;3:PO.18.00211.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim DW, Tiseo M, et al. Exploratory Analysis of Brigatinib Activity in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J Clin Oncol 2018;36:2693-701. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Chow LQ, Barlesi F, Bertino EM, et al. 1478O - Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Ann Oncol 2019;30:v602-v603. [Crossref]
- Zweig JR, Neal JW. Infiltrating the Blood-Brain Barrier in ALK-Positive Lung Cancer. J Clin Oncol 2018;36:2677-9. [Crossref] [PubMed]
- Huber RM, Hansen KH, Paz-Ares Rodriguez L, et al. Brigatinib in Crizotinib-Refractory ALK+ NSCLC: 2-Year Follow-up on Systemic and Intracranial Outcomes in the Phase 2 ALTA Trial. J Thorac Oncol 2020;15:404-15. [Crossref] [PubMed]
- Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Gadgeel S, Shaw AT, Barlesi F, et al. Time To Response In Patients With Advanced Anaplastic Lymphoma Kinase (ALK)-Positive Non-Small-Cell Lung Cancer (NSCLC) Receiving Alectinib In The Phase II NP28673 And NP28761 Studies. Lung Cancer (Auckl) 2019;10:125-30. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405-11. [Crossref] [PubMed]
- Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 2017;35:2613-8. [Crossref] [PubMed]
- Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1-2 trials. Lancet Oncol 2020;21:261-70. [Crossref] [PubMed]
- Yun MR, Kim DH, Kim SY, et al. Repotrectinib Exhibits Potent Antitumor Activity in Treatment-Naive and Solvent-Front-Mutant ROS1-Rearranged Non-Small Cell Lung Cancer. Clin Cancer Res 2020;26:3287-95. [Crossref] [PubMed]
- Cho BC, Drilon AE, Doebele RC, et al. Safety and preliminary clinical activity of repotrectinib in patients with advanced ROS1 fusion-positive non-small cell lung cancer (TRIDENT-1 study). J Clin Oncol 2019;37:9011. [Crossref]
- Baldacci S, Avrillon V, Besse B, et al. Lorlatinib for advanced ALK and ROS1+ non-small cell lung cancer (NSCLC): Efficacy and treatment sequences in the IFCT-1803 LORLATU expanded access program (EAP) cohort. J Clin Oncol 2020;38:9615. [Crossref]
- Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Lin JJ, Shaw AT. Recent Advances in Targeting ROS1 in Lung Cancer. J Thorac Oncol 2017;12:1611-25. [Crossref] [PubMed]
- Okada K, Araki M, Sakashita T, et al. Prediction of ALK mutations mediating ALK-TKIs resistance and drug re-purposing to overcome the resistance. EBioMedicine 2019;41:105-19. [Crossref] [PubMed]
- Li J, Sun R, Wu Y, et al. L1198F Mutation Resensitizes Crizotinib to ALK by Altering the Conformation of Inhibitor and ATP Binding Sites. Int J Mol Sci 2017;18:482. [Crossref] [PubMed]
- Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res 2020;26:2535-45. [Crossref] [PubMed]
- Mezquita L, Swalduz A, Jovelet C, et al. Clinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients With Non-Small-Cell Lung Cancer. JCO Precis Oncol 2020;4:PO.19.00281.
- Horn L, Whisenant JG, Wakelee H, et al. Monitoring Therapeutic Response and Resistance: Analysis of Circulating Tumor DNA in Patients With ALK+ Lung Cancer. J Thorac Oncol 2019;14:1901-11. [Crossref] [PubMed]
- Dagogo-Jack I, Rooney M, Nagy RJ, et al. Molecular Analysis of Plasma From Patients With ROS1-Positive NSCLC. J Thorac Oncol 2019;14:816-24. [Crossref] [PubMed]
- De Mattos-Arruda L, Mayor R, Ng CK, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 2015;6:8839. [Crossref] [PubMed]
- Ying S, Ke H, Ding Y, et al. Unique genomic profiles obtained from cerebrospinal fluid cell-free DNA of non-small cell lung cancer patients with leptomeningeal metastases. Cancer Biol Ther 2019;20:562-70. [Crossref] [PubMed]
- Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol 2018;29:945-52. [Crossref] [PubMed]
- Nevel KS, Wilcox JA, Robell LJ, et al. The Utility of Liquid Biopsy in Central Nervous System Malignancies. Curr Oncol Rep 2018;20:60. [Crossref] [PubMed]
- Offin M, Chabon JJ, Razavi P, et al. Capturing Genomic Evolution of Lung Cancers through Liquid Biopsy for Circulating Tumor DNA. J Oncol 2017;2017:4517834. [Crossref] [PubMed]
- Xu Y, Hu M, Zhang M, et al. Prospective study revealed prognostic significance of responses in leptomeningeal metastasis and clinical value of cerebrospinal fluid-based liquid biopsy. Lung Cancer 2018;125:142-9. [Crossref] [PubMed]
- Li M, Li D, Hou X, et al. Utilizing phenotypic characteristics of metastatic brain tumors to predict the probability of circulating tumor DNA detection from cerebrospinal fluid. J Clin Oncol 2020;38:2507. [Crossref]
- Geraud A, Mezquita L, Auclin E, et al. MA21.09 Tyrosine Kinase Inhibitors' Plasma Concentration and Oncogene-Addicted Advanced Non-Small Lung Cancer (aNSCLC) Resistance. J Thorac Oncol 2019;14:S337-8. [Crossref]
- Luo H, Zhao Q, Wei W, et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci Transl Med 2020;12:eaax7533. [Crossref] [PubMed]
- Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 2017;28:iv84-iv99. [Crossref] [PubMed]
Cite this article as: Aldea M, Vasseur D, Teixidó C, Mezquita L. Central nervous system progression and liquid biopsy in patients with oncogene addicted non-small cell lung cancer treated with ALK/ROS1 inhibitors. Precis Cancer Med 2020;3:25.

