“Lazarus effect” in patient affected by lung adenocarcinoma carrying EGFR, CTNNB1, MET exon 11 and PIK3CA mutations treated with gefitinib
Introduction
Lung cancer is the most commonly diagnosed tumor across the globe (11.6%) and the leading cause of cancer death in both male and female (18.4%) (1).
Molecular characterization became a well-defined diagnostic procedure in patients carrying non-small cell lung cancer (NSCLC) and allows the diagnosis of specific NSCLC sub-categories, the so called “oncogene-addicted” tumors. Patients carrying distinct gene mutations show significant differences in terms of clinical features and therapeutic opportunities (2). In fact, for some driving mutations, development of specific targeted therapies has now revolutionized the treatment of advanced disease.
In particular, EGFR tyrosine kinase inhibitors (EGFR-TKIs) have dramatically improved the outcomes of patients affected by EGFR-mutated lung adenocarcinoma. On the other hand, NGS sequencing (Next Generation Sequencing) also allows the identification of multiple concurrent mutations, whose clinical impact often remains unclear. Currently there are not available treatments for CTNNB1 exon 3 mutations, PIK3CA exon 2 mutations and MET exon 11 mutations; on the other hand, osimertinib is the standard of care for EGFR-mutated metastatic lung adenocarcinoma. Recently, Food and Drug Administration (FDA) approved first line treatment with capmatinib for patients affected with NSCLC carrying MET exon 14 skipping mutations or MET amplifications (more than 10 gene copy number) (3).
We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/pcm-20-32).
Case presentation
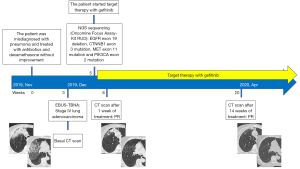
Here we report the case of a 60-year-old lady, never smoker, admitted in pulmonology intensive care unit for acute respiratory distress and hypoxemia in December 2019. In November, she was previously misdiagnosed with pneumonia and treated with antibiotics (piperacillin-tazobactam 18 g daily for 10 days plus levofloxacin 750 mg daily for 10 days) and methylprednisolone (40 mg daily for 10 days) without improvement. EBUS-TBNA was performed and she was diagnosed with metastatic lung adenocarcinoma (stage IV).
During hospitalization, she experienced a worsening of respiratory function and needed high-flows oxygen (FIO2 80%) achieving only a partial correction of hypoxemia, with O2 saturation of 87%. ECOG Performance Status was 3. She was also treated with amoxicillin/clavulanic acid (3 g daily for 1 week), azithromycin (500 mg daily for 10 days) and prednisolone (25 mg daily for 1 week) without clinical improvement.
During the recovery in pulmonology, comprehensive molecular profiling by NGS sequencing (Oncomine Focus Assay, Kit RUO) was performed and showed EGFR exon 19 deletion, CTNNB1 exon 3 mutation, MET exon 11 mutation and PIK3CA exon 2 mutation.
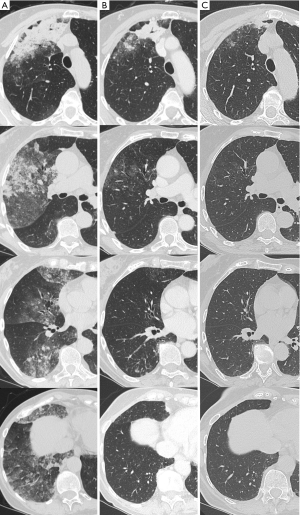
Within few hours, after having obtained NGS results, we started first-line treatment with gefitinib (250 mg daily) achieving an immediate improvement in respiratory function and clinical condition (a so called “Lazarus effect”) while a CT scan performed 7 days after therapy’s start showed a partial response (PR). The CT scan performed after 14 weeks of treatment (April 2020) confirmed the PR (Figures 1,2). The patient is still alive and on treatment with gefitinib. Moreover after 7 months from the diagnosis the patient shows no signs of disease progression.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Discussion
The discovery of EGFR somatic mutations and the availability of potent and selective EGFR tyrosine kinase inhibitors (TKIs) have revolutionized NSCLC’s treatment (4) with impressive response such as the so called “Lazarus effect” (5).
Molecular profiling of NSCLC is currently an established routine practice in patients with advanced disease. Recently, NGS is progressively replacing sequential single gene assessment, leading to the discovery of a significant proportion of patients presenting multiple and concomitant molecular alterations, whose impact on prognosis and response to targeted agents remains often unclear.
Our clinical case showed 4 gene alterations, 3 of whom recognized as pathogenetic (EGFR, CTNNB1 and PIK3CA) and 1 (MET exon 11 mutation) of unclear biological significance.
While the predictive impact of EGFR is now well established, the significance of PIK3CA, MET exon 11 and CTNNB1 is still object of investigation.
Preclinical data of concurrent PIK3CA and EGFR mutations reported how the continuous activation of PI3K signalling by PIK3CA oncogenic mutant was sufficient to abrogate gefitinib-induced apoptosis in EGFR mutated NSCLCs cell line. Clinical studies showed no significant differences in objective response rate, median time to response, TTP, and duration of EGFR-TKI therapy in patients with EGFR and PIK3CA co-mutations. However, median overall survival (OS) was significantly shorter (18.0 vs. 33.3 months), suggesting that the PI3KCA pathway activation can be associated with EGFR-TKI resistance (6).
Regarding β-catenin, previous papers reported its main role in tumorigenesis, especially in the origin and development of lung cancers carrying EGFR resistance mutations. In fact, inhibition of β-catenin or deletion of CTNNB1 (β-catenin encoding gene) has been shown to reduce EGFR-L858R-T790M-mutated lung tumor growth both in vitro and in vivo (7).
Finally, while MET exon 14 skipping mutation is a recognized mechanism of resistance to EGFR-TKIs treatment in 5% to 26% of NSCLCs (8), MET exon 11 mutations are very uncommon and, therefore, their prognostic impact on EGFR-TKI activity in EGFR/MET co-mutated NSCLCs has not yet been assessed.
Conclusions
In clinical practice, the increasing use of NGS allows to identify a discrete proportion of NSCLC patients with multiple and concurrent molecular alterations, whose optimal treatment strategy remains unclear.
Although PIK3CA, CTNNB1 and MET co-mutations are all regarded as negative prognostic factors, being associated with EGFR-TKI resistance, a dramatic response to EGFR-TKI treatment was achieved in our clinical case, thereby suggesting that these co-mutations may act as “passenger” molecular alterations and should not, therefore, affect clinical decisions.
In our opinion, among the strengths of our case report is the rarity of the clinical case. Indeed, our patient presents 4 concurrent mutations: 3 of which (MET, CTNNB1 and PI3KCA) have been shown to be associated with EGFR-TKI resistance in preclinical trials. The improvement of the precision oncology through NGS sequencing allows to identify multiple coexisting mutations, but some of them are adversely prognostic and not “targetable”. Further studies will be helpful to point out which of these mutations acts as prognostic or predictive factors, guiding the therapeutic strategy.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/pcm-20-32
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm-20-32). AA is an unpaid consultant/advisory board member for MSD, AstraZeneca, Novartis, Roche, and Bristol-Myers Squibb. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Barlesi F, Mazieres J, Merlio JP, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet 2016;387:1415-26. [Crossref] [PubMed]
- Wolf J, Seto T, Han JY, et al. Capmatinib (INC280) in METΔex14 -mutated advanced non-small cell lung cancer (NSCLC): Efficacy data from the phase II GEOMETRY mono-1 study. J Clin Oncol 2019;37:9004. [Crossref]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Langer CJ. The "lazarus response" in treatment-naive, poor performance status patients with non-small-cell lung cancer and epidermal growth factor receptor mutation. J Clin Oncol 2009;27:1350-4. [Crossref] [PubMed]
- Eng J, Woo KM, Sima CS, et al. Impact of Concurrent PIK3CA Mutations on Response to EGFR Tyrosine Kinase Inhibition in EGFR-Mutant Lung Cancers and on Prognosis in Oncogene-Driven Lung Adenocarcinomas. J Thorac Oncol 2015;10:1713-9. [Crossref] [PubMed]
- Nakayama S, Sng N, Carretero J, et al. β-catenin contributes to lung tumor development induced by EGFR mutations. Cancer Res 2014;74:5891-902. [Crossref] [PubMed]
- Westover D, Zugazagoitia J, Cho BC, et al. Mechanisms of acquired resistance to first- and second-generation EGFR tyrosine kinase inhibitors. Ann Oncol 2018;29:i10-9. [Crossref] [PubMed]
Cite this article as: Conci N, Dall’Olio FG, Comellini V, Brocchi S, Golfieri R, Ardizzoni A. “Lazarus effect” in patient affected by lung adenocarcinoma carrying EGFR, CTNNB1, MET exon 11 and PIK3CA mutations treated with gefitinib. Precis Cancer Med 2020;3:23.