
Significance of alectinib in anaplastic lymphoma kinase-tyrosine kinase inhibitors for anaplastic lymphoma kinase-positive patients with non–small cell lung cancer
Introduction
The echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase (ALK) gene was identified for the first time in 2007. The ALK gene is rearranged in 3–7% of patients with non-small cell lung cancer (NSCLC) (1,2). ALK-positive NSCLC is exquisitely sensitive to treatment with ALK-tyrosine kinase inhibitors (ALK-TKIs) (1). Crizotinib, a first-in-class ALK-TKI, demonstrated longer progression-free survival (PFS) than standard chemotherapy in a phase III clinical trial (3-5). However, most patients who received crizotinib therapy show disease progression within 1 year of starting therapy (6).
In Japan, we have used crizotinib (5) as a first-generation ALK-TKI, alectinib (7), ceritinib (8) and brigatinib (was not approved in Japan) (9) as second-generation ALK-TKIs, and lorlatinib (10) as a third-generation ALK-TKI. Next-generation ALK-TKI is expected to have improved therapeutic efficacy (10). Several clinical trials showed that alectinib was significantly superior to chemotherapy for crizotinib naïve and treated patients (11) and that alectinib was significantly superior to crizotinib for crizotinib naïve patients (12,13) in terms of PFS, response rate, and toxicity. Moreover, major pathologic response was achieved using alectinib in surgically resected samples, following the use of alectinib, in our previous case (14). On the basis of those results, alectinib is a new standard treatment for otherwise untreated patients with ALK-positive NSCLC. Other clinical trials showed significantly longer PFS and higher overall response rates associated with ceritinib over first- and second-line chemotherapy in crizotinib naïve and treated patients, respectively (8,15). Moreover, some studies reported on the efficacy of ceritinib after alectinib in patients with ALK-positive NSCLC (16,17).
A crizotinib phase I trial reported that the central nervous system (CNS) was the most common site for single organ disease progression in patients (3). It is reported that crizotinib is less effective for metastatic site of the CNS than second-generation ALK-TKIs (5,7,18).
Given the reported efficacy of ALK-TKIs for patients with ALK-positive NSCLC, new generation ALK-TKIs are greatly anticipated. However, clinical trials account for the majority of existing reports. Little is known about the characteristics and clinical results of patients with ALK-positive NSCLC deemed ineligible for clinical trials. Consequently, the efficacy of ALK-TKI use in real-world clinical settings remains unrealized. In order to obtain real-world data from patients with ALK-positive NSCLC, prospective clinical trial-driven data are needed; however, retrospective and real-world data are also needed.
This present study aimed to examine the characteristics, treatments, outcomes, and survival of patients with ALK-positive NSCLC according to real-world data at our institution.
Methods
We retrospectively examined data from all patients administered ALK-TKIs for NSCLC, regardless of treatment duration, and including patients with advanced or recurrent disease and some J-ALEX trial cases, from March 2012 (when crizotinib was first approved in Japan) until October 2018 at our institution.
Baseline clinical characteristics were determined by retrospective review of medical records, including age at the beginning of ALK-TKI administration, sex, tumor histology, treatment history, location of lesion, age at the beginning of ALK-TKI administration, performance status, tumor status [e.g., recurrence case or TNM status based on IASLC TNM staging system (eighth edition)], smoking status, and initial ALK-TKIs that were administered. Instances of postoperative recurrence were diagnosed by CT during follow-up every 6 months. If recurrence was suspected, positron emission tomography (PET) or magnetic resonance imaging (MRI) was conducted as a secondary diagnostic modality to detect brain metastases (BM). Advanced cases were diagnosed at the first visit and staged radiologically using CT, PET, and/or MRI. The specific ALK-TKI used was determined after discussion with the patient; however, after the J-ALEX trial, we generally recommended alectinib. Patients were diagnosed pathologically with NSCLC, and the presence or absence of the ALK mutation was assessed locally by immunohistochemistry and/or fluorescence in situ hybridization (FISH). Specimens for diagnosis were obtained following surgery or transbronchial lung biopsy.
Patients received ALK-TKIs [500 mg/body (crizotinib), 600 mg/body (alectinib), and 450 mg/body (ceritinib), respectively], and if adverse events occurred, treatment was suspended until the adverse events resolved, with subsequent dose reduction. Patients continued treatment until the emergence of progressive disease (PD), development of unacceptable toxicity, or withdrawal of consent. Most patients were hospitalized upon initiation, but, for patients who received alectinib, hospitalization was at the discretion of the attending physician. Tumor lesions were assessed radiologically every 1–3 months. After treatment suspension, some patients selected another ALK-TKI or systemic chemotherapy (e.g., platinum-based doublet regimens) and, if necessary, added local treatment for BM [e.g., stereotactic radiosurgery (SRS) or whole brain radiation therapy (WBRT)], whereas others selected best supportive care.
We assessed overall survival (OS) of all patients, time to treatment failure (TTF) following initial ALK-TKI administration, and the detailed treatment course of each patient. OS was measured from the date of ALK-TKI initiation to the date of death due to any cause or the last survival follow-up. TTF was measured from the first day of initial ALK-TKI intake to the date of discontinuance due to any cause (such as PD or intolerable adverse events), or the last survival follow-up. The objective tumor response was evaluated by CT or PET for extracranial disease and by CT or MRI for BM. Tumor responses were assessed as complete (CR), partial (PR), stable disease, or PD, according to the Response Evaluation Criteria in Solid Tumors guidelines (19).
Survival curves were calculated using the Kaplan–Meier method. Statview version 5.0 (Abacus Concepts, Inc., Berkeley, CA, USA) was used for all statistical analyses.
Results
Patients
Eleven patients received ALK-TKIs for NSCLC, including two cases who were enrolled in the J-ALEX trial, from March 2012 until October 2018 at our institution. Table 1 summarizes patients’ characteristics at baseline, the initiation of ALK-TKI administration, in addition to patients’ demographic and clinical characteristics. Detailed descriptions are provided in Table 2.
Table 1
| Clinical factor | No. of patients |
|---|---|
| Gender | |
| Female | 7 |
| Male | 4 |
| Age, years | |
| Median/average | 65/64 |
| Range | 45–85 |
| Smoking status | |
| Current-smoker | 3 |
| Never smoker | 6 |
| Former-smoker | 2 |
| PS (ECOG) | |
| 0 | 10 |
| 1 | 1 |
| Histology | |
| Adenocarcinoma | 11 |
| Others | 0 |
| Treatment history before ALK-TKI | |
| Yes | 10 |
| Surgery only | 8 |
| CRT and surgery | 1 |
| CTx and surgery | 1 |
| No | 1 |
| Tumor location | |
| Limitation of intrathoracic | 7 |
| Extrathoracic | 4 |
| Initial ALK-TKI | |
| Crizotinib | 2 |
| Alectinib | 9 |
ALK-TKIs, anaplastic lymphoma kinase-tyrosine kinase inhibitors; PS, performance status; CRT, chemoradiotherapy; CTx, chemotherapy.
Table 2
| Case | Age (y)/sex | PS | Smoking status | Treatment history before ALK-TKI | Stage at beginning of ALK-TKI | The type of recurrences | Tumor location | Initial ALK-TKI |
|---|---|---|---|---|---|---|---|---|
| 1 | 81/F | 1 | Current | Surgery for diagnosis | IIIB | – | LN (supraclavicular, mediastinal) | Crizotinib |
| 2 | 45/M | 0 | Former | Surgery | Recurrence after surgery | Local (intra-thoracic) | Thoracic dissemination | Crizotinib |
| 3 | 57/F | 0 | Current | CRT, salvage surgery | Incomplete resection | Local (intra-thoracic) | Mediastinal LN, PM | Alectinib |
| 4 | 46/M | 0 | Never | CTx, salvage surgery | Recurrence after surgery | Local (intra-thoracic) | PM | Alectinib |
| 5 | 67/F | 0 | Never | Surgery | Recurrence after surgery | Local (intra-thoracic) | PM | Alectinib |
| 6 | 85/F | 0 | Never | Surgery | Recurrence after surgery | Both | Mediastinal LN, OSS | Alectinib |
| 7 | 65/M | 0 | Current | Surgery | Recurrence after surgery | Systemic | BRA | Alectinib |
| 8 | 65/F | 0 | Never | None | IIIA | – | Primary lesion | Alectinib |
| 9 | 83/F | 0 | Never | Surgery for diagnosis | IV | – | Abdominal LN | Alectinib |
| 10 | 61/F | 0 | Former | Surgery | Recurrence after surgery | Local (intra-thoracic) | PM | Alectinib |
| 11 | 50/M | 0 | Never | Surgery | Recurrence after surgery | Both | PM, OSS | Alectinib |
PS, performance status, ALK-TKIs, anaplastic lymphoma kinase-tyrosine kinase inhibitors; F, female; M, male; CRT, chemoradiotherapy; CTx, chemotherapy; LN, lymph node; PUL, pulmonary; OSS, ossa; BRA, brain.
Among the 11 patients, the median age was 65 years (range, 45–85 years, mean 64 years), with three patients older than 80 years (27.3%). Ten patients were PS 0, and one patient, who was older than 80 years old, was PS 1. Seven patients were female (63.6%), and all patients were diagnosed with adenocarcinoma. Regarding treatment history before ALK-TKIs, 1 patient with a primary lesion had no treatment history, and 10 patients, all without primary lesions, had previously undergone surgery. Of these, two received salvage surgery. Upon initiation of ALK-TKIs, seven patients exhibited recurrent disease postoperatively, two patients (one underwent salvage surgery and one was for the purpose of diagnosis) were stage III, one patient (the purpose was salvage) was stage IV, and one patient (the purpose was diagnosis) underwent non-curative surgery. For seven patients, tumors were limited to the intrathoracic space (only one had a primary lesion). In four patients, the tumor was extrathoracic. Initial ALK-TKIs included crizotinib (n=2) and alectinib (n=9) (Tables 1,2).
Efficacy
At the time of data cutoff, the median follow-up was 21 months (range, 3.5–57 months). The 4-year OS rate was 71.4% for all patients (Figure 1). Initial treatment outcomes for all patients administered ALK-TKIs are summarized in Table 3. All patients achieved PR or CR. Adverse events were associated with crizotinib for two (out of all patients) and alectinib for only one of the nine patients. Three patients received three types of ALK-TKIs (crizotinib, alectinib, and ceritinib); eight patients received only one type of ALK-TKI (crizotinib, n=1; alectinib, n=7).
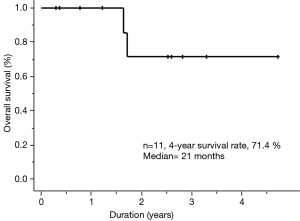
Table 3
| Case | Initial ALK-TKI | Response | Adverse effect | Cause of cessation | Time until cessation of Initial ALK-TKI (days) | Type of ALK-TKIs used | Outcome | OS (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | Crizotinib | PR | Edema, nausea | AE | 325 | One | Deceased | 17.6 |
| 2 | Crizotinib | PR | Edema, renal dysfunction | BM | 804 | Three | Alive | 56.6 |
| 3 | Alectinib | PR | ILD | AE | 26 | Three | Deceased | 20.6 |
| 4 | Alectinib | PR | None | BM | 728 | Three | Alive | 21.6 |
| 5 | Alectinib | CR | None | Ongoing | 657 | One | Alive | 21.6 |
| 6 | Alectinib | PR | None | Ongoing | 949 | One | Alive | 31.2 |
| 7 | Alectinib | PR | None | Ongoing | 917 | One | Alive | 30.2 |
| 8 | Alectinib | PR | None | Personal | 93 | One | Alive | 10.4 |
| 9 | Alectinib | PR | None | Ongoing | 283 | One | Alive | 9.4 |
| 10 | Alectinib | PR | None | Ongoing | 131 | One | Alive | 4.4 |
| 11 | Alectinib | PR | None | Ongoing | 110 | One | Alive | 3.7 |
ALK-TKIs, anaplastic lymphoma kinase-tyrosine kinase inhibitors; OS, overall survival; PR, partial response; CR, complete response; ILD, interstitial lung disease; AE, adverse effect; BM, brain metastases.
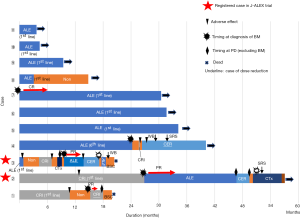
Swimmer pilots are shown in Figure 2. Four patients were treated with crizotinib, including patients who started after the second-line. Cases 3 and 4 discontinued treatment because of adverse effects. In patients treated by crizotinib, three out of four patients (Cases 1, 3, and 4) discontinued because of adverse effects. Ten out of 11 patients were treated with alectinib, including patients who started after the second-line (Case 2). After crizotinib, Case 2 had PR for BM. Three patients were treated with ceritinib who were treated after the third-line. Two of three patients (Cases 2 and 3) were treated for fewer than 4 months, and one of three (case 4) was treated over 1 year. Cases 2 and 3 were enrolled in the J-ALEX trial. Nine of 11 patients had not previously been treated with cytotoxic drugs.
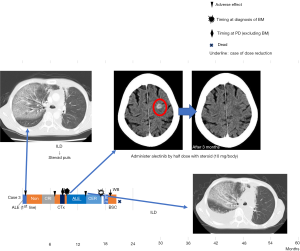
Case 3 developed interstitial lung disease (ILD) following 4 weeks’ administration of ALK-TKIs (Figure 3). We switched her to crizotinib, but soon discontinued treatment secondary to adverse effects. We later administered cisplatin and pemetrexed, but once again discontinued treatment because of adverse effects. We retried alectinib, using a half dose with steroids (10 mg/body). This treatment resulted in good PR for BM. She tolerated this regimen for 4 months, but eventually discontinued treatment because of ILD.
Case 8 was the only patient who underwent salvage surgery after treatment with ALK-TKIs. This case exhibited a major pathological response to alectinib. We believed that the patient was intolerable by chemoradiotherapy because the patient had a mental illness. Therefore, the patient was administered ALK-TKI but not general therapy. This case was previously reported in detail (13).
Five patients developed BM during follow-up. ALK-TKIs were effective on BM for four of five patients (three patients: Cases 2, 3, and 7 were administered alectinib and one patient, Case 1, received crizotinib). Case 7 continued to exhibit good PR for BM for more than 30 months. Cases 2 and 3 experienced recurrent BM after switching to ceritinib from alectinib. Case 4 developed BM following administration of alectinib; however, crizotinib and ceritinib exerted no effect on BM. Consequently, this patient underwent local radiation treatment (RTx) therapy for BM.
Discussion
To the best of our knowledge, this is the only retrospective study of ALK-TKIs in a real-world single institution setting. Prior to this, a little was known about treatment with ALK-TKI in patients with ALK-positive NSCLC from a single institution (20-22); moreover, there are few retrospective studies of ALK-TKIs from the Department of Chest Surgery of the single institution.
In the present study, all cases underwent resection of the primary lesion. Ten out of 11 cases had no primary lesion upon initial treatment of ALK-TKIs. It is important to research the effects of ALK-TKIs in patients with ALK-positive NSCLC without primary lesions in order to better understand the treatment effects on metastatic lesions. Although we only examined 11 cases, the small cohort size enabled us to report detailed information about each respective case. Thus, we were able to obtain real-world data pertaining to ALK-TKI treatment in patients with ALK-positive NSCLC at the Department of Chest Surgery of single institution.
This work produced three major findings. First, our data revealed good treatment outcomes associated with ALK-TKIs in patients with ALK-positive NSCLC. Although our cohort was small, the 4-year OS rate was 71.4% for all patients. This percentage is non-inferior compared with other reports, described in the Introduction section of this study. Ten out of 11 cases were diagnosed ALK-positive NSCLC following examination of surgically resected samples. Abe et al. reported that there was a discordance in ALK gene rearrangement FISH between small biopsy and excision samples in patients with ALK lung cancer (23). In general, almost all reports of patients with ALK-positive NSCLC involve late-stage disease. These patients are not candidates for surgical resection of the primary lung cancer. Our results showed similar clinical outcome to studies that obtained biopsy samples, even though almost all samples were surgically resected specimens.
Second, alectinib produced the best outcomes compared with other ALK-TKIs. Alectinib is a key ALK-TKI. Recent phase III studies found that alectinib was superior to crizotinib in terms of efficacy and toxicity (12,13). Although we examined a small number of subjects, our data revealed similar outcomes. Seven patients, excluding Case 3 who developed ILD, where the initial therapy was alectinib were progression-free and did not have to receive cytotoxic chemotherapy. For Case 3, who had ILD, crizotinib, ceritinib, and cytotoxic chemotherapy were not efficacious. Indeed, for this patient, re-challenge with alectinib plus steroids produced the best outcomes. Moreover, case 8 exhibited a major pathologic response as well as a radiological response following administration of alectinib (14). On the other hand, previous examinations of crizotinib failed to show a good pathological response, despite having achieved a good radiologic response (24,25). For patients with ALK-TKI, salvage surgery after alectinib treatment may be safe and effective for treating initially unresectable ALK-positive NSCLC.
Third, this study showed the effectiveness of ALK-TKI for BM in patients with ALK-positive NSCLC. In generally, alectinib is considered more effective for CNS metastases than crizotinib (5,7,18). However, there are a few repots describing the efficacy of crizotinib for CNS metastases with ALK-positive NSCLC (26). In our study, Case 1 achieved a good CNS response following administration of crizotinib. On the other hand, alectinib also produced good responses for CNS metastases, as demonstrated by Cases 2 and 3. Moreover, other ALK-TKIs (crizotinib and ceritinib) were not effective for CNS metastases that were detected during alectinib treatment, as with Case 4. We believe that initial ALK-TKI selection should favor alectinib, which appears more tolerable and has demonstrated good efficacy as a first treatment for patients with CNS metastases with ALK-positive NSCLC before local treatment, such as RTx or surgery.
There are some limitations to the present study. First, this was a retrospective study and involved only one institution with a small sample size. In addition, although we regularly diagnose tumor response by CT, MRI, and/or PET, the intervals for performing radiographic studies and examination selection were at the discretion of each attending physician. However, we believe our real-world data are nonetheless important. Second, this study took place during a period when alectinib and ceritinib were not approved. Case 1 also might have used other ALK-TKI, if it was now. Third, this study included some cases with limited follow-up, such as Cases 10 and 11. However, because this study took place at only one institution, it was necessary to resister as many cases as possible and we were therefore not strict with the requisite follow-up time.
Conclusions
Alectinib was associated with the best outcomes and efficacy, including for the treatment of BM and toxicity, compared with other ALK-TKIs. Our efficacy data suggested that alectinib produced promising radiological and major pathological responses for patients with ALK-positive NSCLC. Alectinib is an important drug, with efficacy that is comparable with other, currently approved ALK-TKIs. These results suggest that alectinib is well suited as a first-line therapy in these patients.
Acknowledgments
We would like to thank Enago Group (https://www.enago.jp/) for editing a draft of this manuscript.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.12.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Committee of the University of Occupational and Environmental Health Japan approved this study (H26-15). Informed consent was obtained from the patients before the study commenced.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 2012;13:1011-9. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Ou SH, Jänne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Sullivan I, Planchard D. Editorial on the article entitled "brigatinib efficacy and safety in patients with anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer in a phase I/II trial". J Thorac Dis 2016;8:E1287-92. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol 2018;29:1409-16. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw ATALEX Trial Investigators, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Imanishi N, Yoneda K, Tanaka F. at al. Major pathologic response to alectinib in ALK-rearranged adenocarcinoma of the lung. Surg Case Rep 2018;4:19. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci 2018;109:2863-72. [Crossref] [PubMed]
- Yoshida H, Kim YH, Ozasa H, et al. Efficacy of Ceritinib After Alectinib for ALK-positive Non-small Cell Lung Cancer. In Vivo 2018;32:1587-90. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- De Carlo Elisa, Del Savio Maria Chiara, Polesel Jerry, et al. Outcomes of ALK positive lung cancer patients treated with crizotinib or second-generation ALK inhibitor: a monoinstitutional experience. Oncotarget 2018;9:15340-9. [Crossref] [PubMed]
- Metro G, Lunardi G, Bennati C, et al. Alectinib’s activity against CNS metastases from ALK-positive non-small cell lung cancer: a single institution case series. J Neurooncol 2016;129:355-61. [Crossref] [PubMed]
- Itchins M, Hayes SA, Gill AJ, et al. Pattern of care and survival of anaplastic lymphoma kinase rearranged non-small cell lung cancer (ALK+ NSCLC) in an Australian Metropolitan Tertiary Referral Centre: A retrospective cohort analysis. Asia Pac J Clin Oncol 2018;14:e275-82. [Crossref] [PubMed]
- Abe H, Kawahara A, Azuma K, et al. Heterogeneity of anaplastic lymphoma kinase gene rearrangement in non-small-cell lung carcinomas: a comparative study between small biopsy and excision samples. J Thorac Oncol 2015;10:800-5. [Crossref] [PubMed]
- Kaseda K, Watanabe K, Asakura K, et al. Surgical resection of lung adenocarcinoma after crizotinib treatment: report of a case. World J Surg Oncol 2015;13:74. [Crossref] [PubMed]
- Dumont D, Dô P, Lerouge D, et al. Off-Label Use of Crizotinib as a Neoadjuvant Treatment for a Young Patient When Conventional Chemotherapy Gave No Benefits in Stage IIIA Non-Small Cell Lung Cancer. Am J Case Rep 2017;18:890-3. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Clinical impact of crizotinib on central nervous system progression in ALK-positive non-small lung cancer. Lung Cancer 2016;97:43-7. [Crossref] [PubMed]
Cite this article as: Chikaishi Y, Kobayashi K, Matsumiya H, Taira A, Nabe Y, Shinohara S, Kuwata T, Takenaka M, Oka S, Hirai A, Kuroda K, Imanishi N, Ichiki Y, Tanaka F. Significance of alectinib in anaplastic lymphoma kinase-tyrosine kinase inhibitors for anaplastic lymphoma kinase-positive patients with non–small cell lung cancer. Precis Cancer Med 2020;3:3.

