
走向临床应用的循环肿瘤细胞
引言
作为一种致死性疾病,癌症是世界范围内导致死亡的主要原因之一[1]。大部分癌症在早期可以治愈,因此早期检测可增加治疗成功的机会,从而减少与癌症相关的死亡。此外,在癌症监测中,针对微小残留病灶(minimal residual disease,MRD)的实时检测,对临床随访和预后评估也至关重要[2]。
鉴于组织样本获得困难,液体活检,尤其是基于血液的生物样本,为癌症检测和患者的实时随访提供了另一种微创方法[7,8]。常见的来源于血液的检材包括循环肿瘤细胞(circulating tumor cells,CTC)[3]、循环肿瘤DNA(circulating tumor DNA,ctDNA)[4]、细胞外囊泡(extracellular vesicles,EV)[5]和肿瘤驯化的血小板(tumor-educated platelets,TEPs)[6]等。
迄今为止,研究最多的生物检材仍是CTC。这些癌细胞从原发和/或转移部位流入血液和/或淋巴循环,并可能在远端微环境中形成新的(微)转移灶[7]。由于其巨大的潜力,所以,癌症患者CTC分析的临床意义上最近受到了广泛的关注。
CTC可以反映恶性肿瘤不同阶段的突变和异质性,并可作为特定时间癌症进展的直观反映[9]。在临床或影像学征象出现之前,CTC即可出现,这可用于预测转移的存在,亦可能有助于早期发现癌症的发生或复发[2]。
在本综述中,我们讨论了在不同类型癌症中开展的重要临床试验,以及这些研究是怎样指导临床医生进行精准医疗活动的。
CTC当前的临床应用
较高数量的CTC与较差的预后密切相关[10]。同样,在治疗过程中持续CTC的存在,可以预测患者较短的总生存期(overall survival,OS)。因此,可对CTC进行计数和表征,以监测疾病的进展和对治疗的反应性(耐药性)。
值得注意的是,虽然CTC计数的可行性较高,但它们在外周循环中的数量很少,同时由于CTC表达的分子异质性,使得其分离和表征非常具有挑战性。因此,CTC尚未被全面应用临床常规测试。已经或正在进行的多项试验研究,均是为了证明CTC的临床相关性,以期将这种生物标志物的真正潜力完全转化,并最终作为临床判断的依据。
对于癌症患者的CTC检测,唯一经证实具有临床有效性[11]并获得食品和药物管理局(the Food & Drugs Agency,FDA)批准的方法是CellSearch®系统(Menarini Silicon Biosystems,Inc;Bologna,意大利)。该方法根据上皮标志物(例如EpCAM、PanCK)的表达,进行捕获并检测CTC。简而言之,第一步,抗EpCAM抗体修饰的磁珠用于富集;第二步,通过针对细胞角蛋白和核酸的荧光染色来观察CTC,从而将其与CD45+的白细胞区分开来[12]。
该技术现已用于乳腺癌[13]、结直肠癌(colorectal cancer,CRC)[14]、前列腺癌[15]、小细胞肺癌(small cell lung cancer,SCLC)[16]、非小细胞肺癌(non-small cell lung cancer,NSCLC)[17]和胰腺癌[18]相关的CTC检测。CTC的预后作用,也已在多种其他癌症中得到证实[10,19,20],我们将针对上述恶性肿瘤的一些最重要临床见解进行描述(图1)。
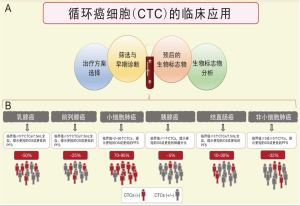
对于转移性乳腺癌(metastatic breast cancer,MBC),可在约50%的患者中检测到CTC[10]。Bidard等对来自17个不同中心的1 944个患者进行汇总分析,证明当每7.5 mL血液中≥5个CTC时,提示更短的OS和更短的无进展生存期(progression-free survival,PFS),从而确定了CTC计数的临床有效性[11,13],并且已经证明该CTC与远处骨髓中存在的肿瘤细胞无关[21]。同样,使用CTC可针对cM0(i+)的乳腺癌进行分期,目前已为WHO乳腺癌分类第4版[22]和美国癌症联合委员会(the American Joint Committee on Cancer,AJCC)癌症分期手册第8版所收录[23]。此外,Cristofanilli等提议,根据每7.5 mL血液中≥5个CTC的临界值,IV期MBC可进一步分为“惰性”和“侵袭性”两个亚类;在对18个队列的汇总分析中,他们证明CTC计数独立于临床和分子变异[24]。除了CTC在乳腺癌分期和预后中的作用外,在过去十年中,还进行了几项随机前瞻性临床试验,通过对比,证明了CTC检测在MBC管理中的临床效用[19]。尽管CTC计数在预后评估中的作用已得到明确支持,但是,Smerage等在一项介入性临床试验中(SWOG S0500;NCT00382018),对一线化疗后21 d仍存在CTC数量持续高(7.5 mL血液中≥5个CTC)的患者,由于未能判断化疗药物导致细胞计数改变,从而未能证明证明CTC计数的临床实用性,尽管CTCs计数的预后作用得到了明确的支持[25]。然而,值得注意的是,针对这类MBC患者,目前临床尚无有效治疗策略,因此无论使用何种生物标志物,在这种情况下,最后得到的数据都是相同的,即“没有临床相关性”。最近,正在进行中的STIC-METABREAST试验(NCT01710605),试图证明在HER2阴性和激素受体阳性的MBC中,CTC的使用与驱动所选择的临床化疗决策(每7.5 mL血液中≥5个CTC)或内分泌治疗(每7.5 mL血液中<5个CTC)在具有临床上的相关性;该干预性临床试验应该可以证明CTC应用的临床实用性[26]。然而,由于CDK4/6抑制剂的引入,这些MBC的标准治疗方案在试验期间发生了变化[27]。
在转移性结直肠癌(metastatic CRC,mCRC)中,更多数量的CTC与较差的预后密切相关[14]。不同研究之间的CTC计数差异很大,在治疗前的基线状态,发现仅有10%~30%的患者是CTC阳性[14]。有人提出,与乳腺癌或前列腺癌相比,这种CTC的低值是肝脏的功能性过滤的结果[28]。以每7.5 mL血液中≥3个CTC为临界值,已被证明是用于预测OS和PFS的良好指标[29,30];随后,出于相同目的,其他研究甚至使用了每7.5 mL血液中≥1个CTC作为临界值[14,31]。目前,没有明确定义的通用临界值,因此也尚未证明CTC在mCRC中的临床实用性。
在SCLC中,70%~95%的患者可检测到CTC[16]。此外,与其他癌症类型相比,CTC的数量明显更高,这支持了CTC检测在SCLC中的应用。一项多中心前瞻性研究表明,少量CTC的存在可以预测SCLC的分期(局限期或广泛期) [32]。此外,在第一个化疗周期后出现更多数量的CTC与较差的OS和PFS密切相关[10,32]。但同为SCLC,其临界值在不同研究之间变化很大(从>2到>50个不等)[10,16]。
与SCLC相比,NSCLC患者中,出现CTC的患者相对较少,所检测到的CTC数量亦较少。据Krebs等人报道,只有32%的转移性疾病患者每7.5mL血液中出现≥2个CTC[33]。尽管如此,已经证明≥2或≥5个CTC的患者与最差OS和PFS相关[17]。基线≥5个CTC的临界值,与计算机断层扫描(computer tomography,CT)显示对治疗的反应相一致[34],并且还与对酪氨酸激酶抑制剂的不利反应有关[35]。
同样,在胰腺癌中,使用CellSearch®系统对CTC的检出率相当低。据报道,可捕获CTC(临界值≥1个CTC)患者的比例低至5%[18],然而,该患者亚组与较短的OS和较差的肿瘤分化相关[18]。
在前列腺癌中,已在局限性和转移性患者中评估了CTC的应用价值。在局限性前列腺癌中,可能会因CTC在血液中的数量较少而导致CTC的检测受限[36]。然而,Kuske等在根治性前列腺切除术后的患者中,通过使用三种不同的方法检测CTC,提示CTC计数可作为MRD的标志物[37]。目前,涉及局限性前列腺癌中CTC的研究仍然很少[36],但欧洲PROLIPSY项目运行的新的临床试验,将可能回答我们是否可以使用液体活检来诊断前列腺癌,并评估其侵袭性。同时,在转移性前列腺癌中,已有几项临床试验使用每7.5 mL血液≥5个CTC作为临界值标准,并证明其与较短OS相关,从而证明了其临床有效性[36]。此外,Scher等也证明了作为早期治疗反应标志物,CTC计数优于前列腺特异性抗原(PSA)[15,36],并且有人建议将其用作临床试验中OS的替代终点[38]。
目前,正在进行的一项临床试验证明了在转移性去势抵抗性前列腺癌中计数CTC的临床实用性(TACTIK研究;NCT03101046),确立每7.5 mL全血中≥5个CTC的临界值作为对多西紫杉醇耐药性的标志,并作为调整化疗方案的指征。此外,在CTC中检测雄激素受体变体7(AR-V7),已同样显示出其临床价值,并可用于评估内分泌治疗反应和预测预后。Armstrong等在一项多中心前瞻性临床试验中(PROPHECY研究;NCT02269982),基于两种不同方法检测CTC中mRNA,评估了AR-V7检测的临床意义。结果表明,当患者接受抗雄激素治疗时,CTC中AR-V7变体的阳性与较短的OS和PFS相关,表明改变治疗方案或可使这些患者受益[39]。此外,完整CTC可视化技术的应用,可以提供迄今为止其他方法均无法提供的相关细胞学信息;例如,Scher等报道,只有定位于核的AR-V7变体与指导治疗相关[40-42]。
CTC的检测不仅限于上述恶性肿瘤。事实上,在最近的临床试验中(CIRCUTEC研究:NCT02119559),Garrel等通过三种不同的技术,证明了血液中CTC的数量与头颈部鳞状细胞癌治疗的早期反应相关[20]。目前,为确定针对不同癌症类型的治疗新方案,更多的临床试验正在进行中。
CTC领域的未来技术和应用
目前,除了CTC计数之外,学者们还做了许多努力试图证明CTC的临床相关性:例如在CTC中检测PD-L1,以预测对免疫检查点抑制剂疗法(如nivolumab)的反应;例如在乳腺癌、头颈部鳞状细胞癌或NSCLC的研究中,通过免疫和分子方法评估了PD-L1的表达[43-45]。相似地,亦可检测可被靶向治疗的其他生物标记物和突变(例如,雌激素受体、HER2和KRAS状态)[2,19]。此外,血液中CTC簇及其“循环微环境”的计数和分析与患者的预后结果相关,并且特定的表观遗传变化与这些簇也高度相关[46-48]。CTC的另一个有前景的用途是在MRD的背景下,针对性治疗后CTC的数量可以作为复发的标志物[2]。然而,直至撰写本文时,除了临床转化试验外,这些方法都没有完全纳入临床实践。
另外,学者们还开发了其他用于CTC富集、捕获和计数的创新方法。如Parsortix™ PC1系统(ANGLE North America, Inc., King of Prussia, PA, USA)就是一种基于尺寸和变形性捕获CTC的微流体装置,该方法具有捕获那些低表达上皮标志物CTC的理论优势[49]。为了证明该方法在MBC中的有效性,目前正在进行一项临床试验(ANG-002;NCT03427450),以期证明其在不同MBC评估类型中的实用性。其他方法,包括基于上皮细胞特异性的不同标记、光声、电荷、代谢及其功能的方法等,均尚未经过充分的试验证实其可用于临床[50-53]。此外,采用不同的技术设备及不同的方法,可用来研究被正常造血细胞包围的稀少CTC:例如从微流体芯片设备[50,54,55]到筛选大量血液的白细胞去除系统[56],独立地基于细胞表面标志物和/或生物力学特性,这些方法也将为捕获早期阶段的CTC或CTC簇的分析提供更广泛的方法[57,58]。另一个正在研发中的方向是单细胞CTC的分析,这些分离和分析方法可提供基因组、转录组、蛋白质组和分泌组水平等不同层面的信息,从而大大扩展CTC的临床应用[2]。
结论
作为可实时液体活检的代表,CTC提供了一种很有前途的肿瘤学个体化治疗方法。CTC在日常临床实践中的普遍使用尚未成为现实,过去曾因其在循环中的数量极少以及在癌症进展过程中的表型变化,导致其应用受阻。尽管如此,借助稳定、可重复的技术进行计数,在特定的临床环境中,CTC检测可提供非常有价值的信息。可以确定的是,在未来更多的临床实践中,CTC的检测将提供更多可指导临床实践的治疗和临床管理信息,这只是时间问题而已。此外,当前非常活跃的开发检测和表征CTC的若干可选择的技术,正在创造并丰富该领域的内涵,在该领域中,可分析CTC的基因组、转录组、蛋白质组和分泌组,并为治疗提供预后评估、治疗状态和转归预测等相关信息。因此,已经启动了诸如European CANCER-ID、欧洲液体活检学会(ELBA)、欧洲液体活检协会(ELBS)工作网,以及美国BloodPAC等项目,来应对眼下的这些挑战。
Acknowledgments
Funding: This work was supported by (I) ELBA project received funding from the European Union Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 765492, (II) CANCER-ID, an Innovative Medicines Initiative Joint Undertaking under grant agreement no. 115749, resources of which are from the European Union’s Seventh Framework Program (FP7/2007-2013) (www.cancer-id.eu) and EFPIA companies’ in-kind contribution, (III) the National Institute of Cancer (INCa, http://www.e-cancer.fr).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.11.07). This project has received funding from the European Union Horizon 2020 Research and Innovation program under the Marie Skłodowska-Curie grant agreement No 765492. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease - latest advances and implications for cure. Nat Rev Clin Oncol 2019;16:409-24. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Circulating tumor cells: liquid biopsy of cancer. Clin Chem 2013;59:110-8. [Crossref] [PubMed]
- Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem 2015;61:112-23. [Crossref] [PubMed]
- Sheridan C. Exosome cancer diagnostic reaches market. Nat Biotechnol 2016;34:359-60. [Crossref] [PubMed]
- Best MG, Sol N, Kooi I, et al. RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 2015;28:666-76. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010;16:398-406. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov 2016;6:479-91. [Crossref] [PubMed]
- Brown HK, Tellez-Gabriel M, Cartron PF, et al. Characterization of circulating tumor cells as a reflection of the tumor heterogeneity: myth or reality? Drug Discov Today 2019;24:763-72. [Crossref] [PubMed]
- Riethdorf S, O’Flaherty L, Hille C, et al. Clinical applications of the CellSearch platform in cancer patients. Adv Drug Deliv Rev ;2018125:102-21. [PubMed]
- Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15:406-14. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer 2015;15:202. [Crossref] [PubMed]
- Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009;10:233-9. [Crossref] [PubMed]
- Foy V, Fernandez-Gutierrez F, Faivre-Finn C, et al. The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl Lung Cancer Res 2017;6:409-17. [Crossref] [PubMed]
- Lindsay CR, Blackhall FH, Carmel A, et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur J Cancer 2019;117:60-8. [Crossref] [PubMed]
- Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol 2013;24:2057-61. [Crossref] [PubMed]
- Cabel L, Proudhon C, Gortais H, et al. Circulating tumor cells: clinical validity and utility. Int J Clin Oncol 2017;22:421-30. [Crossref] [PubMed]
- Garrel R, Mazel M, Perriard F, et al. Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: the CIRCUTEC prospective study. Clin Chem 2019;65:1267-75. [Crossref] [PubMed]
- Bidard FC, Vincent-Salomon A, Sigal-Zafrani B, et al. Prognosis of women with stage IV breast cancer depends on detection of circulating tumor cells rather than disseminated tumor cells. Ann Oncol 2008;19:496-500. [Crossref] [PubMed]
- Lakhani S, Ellis I, Schnitt S, et al. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press, 2012.
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer Publishing, 2017.
- Cristofanilli M, Pierga JY, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 2019;134:39-45. [Crossref] [PubMed]
- Smerage JB, Barlow WE, Hortobagyi GN, et al. Circulating tumor cells and response to chemotherapy in metastatic breast cancer: SWOG S0500. J Clin Oncol 2014;32:3483-9. [Crossref] [PubMed]
- Bidard FC, Jacot W, Dureau S, et al. Abstract GS3-07: Clinical utility of circulating tumor cell count as a tool to chose between first line hormone therapy and chemotherapy for ER+ HER2-metastatic breast cancer: results of the phase III STIC CTC trial. San Antonio: 2018 San Antonio Breast Cancer Symposium, 2019. doi:
10.1158/1538-7445.SABCS18-GS3-07 . - Shah M, Nunes MR, Stearns V. CDK4/6 inhibitors: game changers in the management of hormone receptor-positive advanced breast cancer? Oncology 2018;32:216-22. [PubMed]
- Denève E, Riethdorf S, Ramos J, et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin Chem 2013;59:1384-92. [Crossref] [PubMed]
- Burz C, Pop VV, Buiga R, et al. Circulating tumor cells in clinical research and monitoring patients with colorectal cancer. Oncotarget 2018;9:24561-71. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. [Crossref] [PubMed]
- Seeberg LT, Waage A, Brunborg C, et al. Circulating tumor cells in patients with colorectal liver metastasis predict impaired survival. Ann Surg 2015;261:164-71. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Yang B, Qin A, Zhang K, et al. Circulating tumor cells predict prognosis following tyrosine kinase inhibitor treatment in EGFR-mutant non-small cell lung cancer patients. Oncol Res 2017;25:1601-6. [Crossref] [PubMed]
- Maas M, Hegemann M, Rausch S, et al. Circulating tumor cells and their role in prostate cancer. Asian J Androl 2017; [Epub ahead of print]. [PubMed]
- Kuske A, Gorges TM, Tennstedt P, et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci Rep 2016;6:39736. [Crossref] [PubMed]
- Heller G, Fizazi K, McCormack R, et al. The added value of circulating tumor cell enumeration to standard markers in assessing prognosis in a metastatic castration-resistant prostate cancer population. Clin Cancer Res 2017;23:1967-73. [Crossref] [PubMed]
- Armstrong AJ, Halabi S, Luo J, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol 2019;37:1120-9. [Crossref] [PubMed]
- Scher HI, Lu D, Schreiber NA, et al. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol 2016;2:1441-9. [Crossref] [PubMed]
- Pantel K, Hille C, Scher HI. Circulating tumor cells in prostate cancer: from discovery to clinical utility. Clin Chem 2019;65:87-99. [Crossref] [PubMed]
- Scher HI, Graf RP, Schreiber NA, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol 2017;71:874-82. [Crossref] [PubMed]
- Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 2015;9:1773-82. [Crossref] [PubMed]
- Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol 2017;28:1923-33. [Crossref] [PubMed]
- Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced non-small cell lung cancer patients treated with nivolumab. Lung Cancer 2018;120:108-12. [Crossref] [PubMed]
- Balakrishnan A, Koppaka D, Anand A, et al. Circulating tumor cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep 2019;9:7933. [Crossref] [PubMed]
- Szczerba BM, Castro-Giner F, Vetter M, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019;566:553-7. [Crossref] [PubMed]
- Gkountela S, Castro-Giner F, Szczerba BM, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 2019;176:98-112.e14. [Crossref] [PubMed]
- Miller MC, Robinson PS, Wagner C, et al. The ParsortixTM cell separation system-A versatile liquid biopsy platform. Cytometry A 2018;93:1234-9. [Crossref] [PubMed]
- Tang Y, Wang Z, Li Z, et al. High-throughput screening of rare metabolically active tumor cells in pleural effusion and peripheral blood of lung cancer patients. Proc Natl Acad Sci U S A 2017;114:2544-9. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer 2014;14:623-31. [Crossref] [PubMed]
- Galanzha EI, Menyaev YA, Yadem AC, et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci Transl Med 2019; [Crossref] [PubMed]
- Alix-Panabières C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res 2012;195:69-76. [Crossref] [PubMed]
- Cho H, Kim J, Song H, et al. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018;143:2936-70. [Crossref] [PubMed]
- Sequist LV, Nagrath S, Toner M, et al. The CTC-chip: an exciting new tool to detect circulating tumor cells in lung cancer patients. J Thorac Oncol 2009;4:281-3. [Crossref] [PubMed]
- Kim TH, Wang Y, Oliver CR, et al. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat Commun 2019;10:1478. [Crossref] [PubMed]
- Castro J, Sanchez L, Nuñez MT, et al. Screening circulating tumor cells as a noninvasive cancer test in 3388 individuals from high-risk groups (ICELLATE2). Dis Markers 2018;2018:4653109. [Crossref] [PubMed]
- Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014;158:1110-22. [Crossref] [PubMed]

李超
教授。温州医科大学附属第二医院基础医学研究中心主任,休斯顿卫理公会医院博士后,申请主持国家及地方项目多项,发表SCI论文30余篇,H-index 24。申请获批中国发明专利5项,专利转化并医疗器械注册证2项(III类)。(更新时间:2021/7/25)

卓文磊
陆军军医大学新桥医院肿瘤科副主任, 副主任医师,副教授, 国家公派留美博士后, 硕导,从事肿瘤科医疗、教学和科研工作二十余年。任中国抗癌协会肿瘤营养专委会委员等学术职务,持国家自然科学基金项目2项,以第一(通讯)作者发表SCI论文30余篇。(更新时间:2021/7/25)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Eslami-S Z, Cortés-Hernández LE, Alix-Panabières C. Circulating tumor cells: moving forward into clinical applications. Precis Cancer Med 2020;3:4.

