Training and precision surgery in VATS lobectomy
Introduction
Video-assisted thoracic surgery (VATS) for lung cancer was introduced in 1991 (1). Since then VATS lobectomy has become an accepted procedure and emerged as gold standard for treatment of lung cancer worldwide. VATS offers several advantages, including low morbidity, shorter hospitalization, decreased pain, better outcome and better tolerance for adjuvant chemotherapy (2,3). To avoid and overcome substantial complications, thoracic surgeons must develop competence and master VATS to a precision level, where the physicians are permitted to perform procedures independently. They must have extensive knowledge and skills to operate on patients. Developing competence and then proficiency in VATS lobectomy, not only requires enough procedures to achieve consistent safety and efficacy, but also requires qualitative upsurge in knowledge, new skills and performance. The novice surgeons should be mentored and supervised appropriately until they demonstrate skills required for competency and then proficiency in VATS procedures.
There are different opinions about the caseload, time and other program requirements to achieve competence and proficiency in VATS. Some suggest that trainees must perform more than 100 minor procedures to get familiar with the surgical instruments and basic VATS skills before undertaking a VATS lobectomy (4,5). Furthermore, 25 lobectomies per year are suggested to complete learning curves and retain proficiency (4,5). Would a threshold of 25 or 50 VATS lobectomies ensure that a surgeon is competent? Should a VATS surgeon have thoracotomy experience?
There are several un-answered questions about learning curves, VATS programs, the role of simulation in VATS, learning VATS under supervision, assessment tools, certification etc.
The aim of the current review was to get a narrative overview of the different VATS programs, learning curves, VATS assessment tools, role of simulation in VATS and to explore the existing evidence of training models and their efficacy.
Materials and methods
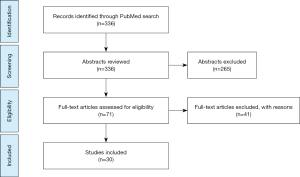
An electronic search was performed in PubMed. Articles published before April 2019 were screened and included if considered relevant. To achieve maximum sensitivity of search strategy and identify all trials, we used the following the terms: training VATS lobectomy, learning curve VATS lobectomy, VATS certification, VATS non-technical skills and VATS simulation. All the studies including VATS lobectomies and VATS learning curves published in English were included. The references of all retrieved articles were reviewed for further identification of any relevant studies. We excluded studies that we did not find directly relevant for this study (Figure 1).
Training and precision
Procedural expertise exhibited by a VATS surgeon is difficult to designate and classify. To qualify and practice VATS independently, it is required that physicians demonstrate competence and have the ability to perform a procedure safely and effectively. To develop competency and efficiency in VATS there is a need for structured education programs. There are many different methods to learn VATS. Surgeons can observe other surgeons, read books, see videos, read anatomical atlases, attend national and international thoracic surgery courses and seminars, perform simulation training etc.
Learning curve and preparation
Several factors influence learning curves, such as the potential number of surgeries, size of center and time between surgeries, preparation before surgery and patient selection. There is a difference in learning curve between a self-taught surgeon and a surgeon under supervision of experienced VATS surgeons. Konge et al. described that the learning curve can be overcome, even with limited prior experience in thoracotomy, with thorough preparation of trainees and training on selected patients under close supervision (6). A trainee who had performed 14 lobectomies by thoracotomy was enrolled in a training program. The preparatory phase included VATS master classes with hands on practice on live pigs and a VATS lobectomy course with a validated lobectomy model and observation of VATS lobectomies both on video and in the operating theatre. The trainee practiced on a simulator, on pigs and performed more than 100 minor VATS procedures and observed more than 100 VATS lobectomies. The preparatory phase was followed by clinical practice, where suitable patients were selected, in regard to factors such as tumor localization, tumor size and absence of major comorbidities. The trainee performed 29 VATS lobectomies in a 12 months’ time under supervision of an experienced VATS surgeon. None of the operations were converted to open thoracotomy and per-operative and post-operative results were acceptable.
Yao et al. investigated the learning curve by using a multidimensional method and compared the learning curve groups with respect to perioperative and clinical outcomes in a retrospectively reviewed prospective study (7). Sixty-seven patients underwent VATS lobectomy by one surgeon and data was analyzed by a moving average and the cumulative sum (CUSUM) method. CUSUM is a sequential analysis method, which monitors the change detection and assume that a process is stable and under control. Their result showed that peak point for operation was at their 26.th case, meaning that their learning curve for VATS lobectomy required 26 cases. Li et al. used the same method to analyze the learning curve of VATS lobectomy (8). They evaluated the development of proficiency in VATS lobectomy using CUSUM analysis. They concluded, to achieve proficiency in performing VATS lobectomy requires 100 to 200 cases and to procure consistency requires more than 200 cases. This result represents the learning curve of a single surgeon with five years of experience in thoracic surgery.
The role of simulation in VATS surgery
A wide range of simulations are developed to increase patient safety by improving novice surgeons’ cognitive and procedural skills in VATS before they can operate on patients.
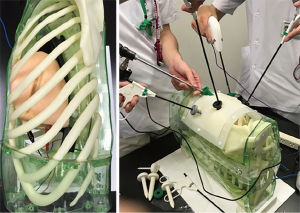
Three different types of simulations modalities are available: Dry lab, wet lab and virtual reality simulation. Dry lab simulators such as Box trainers increase the skills and enable trainees to perform and learn basic procedural skills (Figure 2). The haptic feedback is preserved and creates high fidelity for VATS training (10). Wet lab simulators using the pig model are very sophisticated and create a realistic setting, but are very expensive and bear ethical issues, anatomical difference, difficulties in anesthesia and have poor cardiopulmonary reserves (11). Using porcine heart-lungs tissue blocks from pigs are low cost, accessible, easy-to-prepare and reproducible tissue simulator, that can be effective in instruction to surgeons with different level of operative experience (12).
Virtual reality simulators on the other hand create a realistic setting, where trainees can develop their basic skills such as; hand-eye coordination, depth perception, movement of instruments in a repetitive manner and out of theatre environment where the trainees can achieve surgical competence before operating on patients. Simulators accelerate the learning curve (13).
Different types of virtual reality simulators such as Lap Mentor™ (3D Systems, California, US) or LapSim™ (Surgical Science, Gothenburg, Sweden) are available (Figure 3). The validity of simulation-based assessment and the effect of simulation-based training are evaluated and investigated by Jensen et al. and Bjurström et al. (13-15).
The assessment of competency
The assessment of competence in VATS is discussed in several studies. Konge et al. created an assessment tool consisting of 10 items by an expert group of physicians (16). Fifty consecutive thoracoscopic wedge resections were included and assessed blindly by two experts. They concluded that this tool for assessing performance in thoracoscopy was reliable and valid and can offer structured feedback to trainees and be used to evaluate new teaching curricula. Jensen et al. used a Delphi method and developed a novel assessment tool for VATS lobectomy based on a large number of internationally recognized VATS expert’s consensus (17). The VATSAT (video-assisted thoracoscopic surgery assessment tool) consists of eight items. These items can support learning VATS lobectomy by providing structured feedback and help the supervisors identify when the trainees have acquired competencies for independent performance. The validity evidence for VATSAT was provided by Petersen et al. in a Danish nationwide study (18). They recorded 60 lobectomies performed by 18 thoracic surgeons of different level of experience; expert, intermediate and beginners. The videos were rated using VATSAT and solid evidence of validity was provided. They concluded that VATSAT could be an important aid in future training and certification of thoracic surgeons. The theoretical knowledge acquisition for VATS lobectomy shows that cognitive skills gained through theoretical testing support technical performances (19). It can also stimulate learning and encourage self-study. Savran et al. developed and gathered validity evidence for a theoretical test on VATS lobectomy consisting of multiple-choice questions (20). Technical skills in VATS surgery are dependent on cognitive social and personal resource skills that contribute to safe and efficient task performance and can be defined as Non-technical skills. VATS surgeons are dependent on their team. Team training with a shared mental model of the operation is an important issue. Gjeraa et al. identified non-technical skills for VATS lobectomy (21).
Their study identified six non-technical models. These findings contribute to three important shared mental model constructs; Planning and preparation, risk assessment, and leadership. VATS surgery is team-work and it needs planning, preparation, and risk assessment before each procedure.
Selection of patients
Accurate patient selection is crucial for a safe learning curve in VATS lobectomy. Size and location of tumor, patient comorbidity and frailty are important to ensure a low conversion rate and morbidity rate. Amore et al. recommend different steps in the development of a VATS lobectomy program (22). They recommend careful selection of patients in the beginning. Petersen et al. described in a prospective study that VATS lobectomy can be taught safely with careful selection of patients in a surgical institution experienced in VATS lobectomy (23). Consecutive VATS lobectomies were performed by two consultants; one a self-taught experienced VATS surgeon and one consultant in training for VATS lobectomies. The training consultant performed 200 minor VATS procedures prior to performing supervised VATS lobectomies. In the study period, 150 patients were operated by the experienced surgeon and 47 VATS lobectomies by the consultant in training. The patients for the consultant in training were selected, where majority of patients had early-stage lung cancer. There was no significant difference in patient outcome between VATS lobectomies performed by the experienced consultant and the training consultant, but the procedural time for the consultant in training was significantly longer.
Which programs are available and should be offered?
Efficacy of different training programs are compared in several studies but there is no consensus about which training modality is superior to other.
Konge et al. presented a “four step approach” to a medical simulation program (24). These four steps include: I. Theoretical preparation; II. On-site introduction to the simulation training assisted; III. Self-regulated practicing of the procedure; and IV. End of simulation training certification. They recommend that novice thoracic surgeons undergo mandatory VATS training, including simulation- based training. Training should proceed until competency has been demonstrated using valid assessments methods.
Ferguson et al. concluded that VATS lobectomy can be safely taught to trainee surgeons with no increase in intraoperative blood loss, morbidity, mortality or postoperative stay, but increase in operating time (25). They observed an increase in experience during the development and establishment of a VATS lobectomy program and they recommend that training programs should be coordinated at a national level to aggregate experience. Wan et al. demonstrated that VATS lobectomy can be taught to trainees supervised by experienced surgeons (26). In a prospective study they compared the results of VATS lung resection between experienced thoracic surgeon and surgical trainees under supervision. One hundred eleven patients with clinical stage I and II lung cancer underwent different lung resections in four years. Fifty-one of the procedures were performed by experienced consultant and 60 by trainees under supervision. One patient in the consultant group and 3 in the trainees’ group were converted to open surgery due to bleeding from the pulmonary artery. The trainees spent more time in the operating room compared to experienced VATS surgeons, but there were no significant differences in intraoperative or post-operative complications and outcomes.
Reed et al. concluded that stepwise plan for introduction of a VATS lobectomy training program would facilitate safe learning of the technique (27). They performed 202 lobectomies in a four years period, in which 97 procedures were performed by thoracotomy and 105 were performed thoracoscopic. The number of thoracoscopic procedure increased from 18% in the first quartile to 82% in fourth quartile. This shows that VATS can be achieved safely if a stepwise transition is evoked. Sandri et al. described VATS lobectomy program from a trainee perspective (28). They identified three distinct issues; I. Stepwise approach to VATS lobectomy and standardization of teaching; starting with holding camera and under supervision making the ports to pulmonary ligament dissection and hilum exposure. Then advancing once experience is gained, and the trainee is sufficiently confident the next step will include vessels and bronchus dissection, gradually lobectomy and lastly lymphadenectomy. II. Off-theatre independent training; such as reviewing videos, black boxes and virtual reality simulations. III. Evaluation and certification; they suggest that as an end point to any educational program; assessment of competence and certification should be taken under consideration. Does the previous surgical experience effect the learning curve?
The new thoracic surgeons will not have the ability to start their career with thoracotomy, because of less procedures are performed by thoracotomy. Bille et al. and Konge et al. found that the number of open procedures did not impact intraoperative nor post-operative outcomes (6,29). The question is, how long does it take for a novice surgeon to learn and master VATS lobectomy compared to an experienced surgeon. Ra et al. examined 38 pulmonary lobectomies performed by a single surgeon without VATS lobectomy experience (30). The surgeon performed 100 lobectomies via conventional thoracotomy. They concluded that, it takes six months for a surgeon without experience to reliably perform VATS lobectomy. Out of 38 lobectomies 14 lobectomies were by VATS, 14 lobectomies by thoracotomy and 10 were converted to open thoracotomy. They had a high conversion rate, but the number of VATS lobectomy increased by time.
Conclusions
We have reviewed the recent literature on VATS lobectomy. Several studies show that there is a need for a structured training program to achieve precision. We are moving from an apprenticeship model based on a fixed time or a certain number of procedures to competency-based learning including simulation-based training. We suggest that all thoracic trainees should pass four phases consisting of theory, simulation, supervised clinical training, and independent clinical practice. Each phase should be completed with certification. (I) Theoretical phase; Reading books, articles, attending conferences, and completing multiple choice questions. (II) Off theatre preparatory phase; Starting with virtual reality simulation to learn the basic skills followed by dry and wet lab to get familiar with the real instruments and the feeling of real tissue. (III) In theatre preparatory phase; Stepwise learning, where the trainee starts with placing ports, performing minor VATS procedures; such as wedge resections, pleura biopsies etc. Once sufficient experience is gained and the trainee performance is consistent, VATS lobectomy procedures can be performed under supervision of a VATS expert until the trainee demonstrates skills and outcomes required for competency and then proficiency. (IV) Independent practice; we suggest that every end point of any educational program, evaluation, assessment of competence, should be completed with certification. It is important to continuously monitor per-operative and post-operative results and video records of each performance can be considered for evaluation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Marcelo F. Jimenez for the series “Precision Surgery for Lung Cancer” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.05.02). The series “Precision Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. RHP reports speaker fee from Medtronic. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomized controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind WR, et al. Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 2007;83:1245-9; discussion 1250. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- McKenna RJ Jr. Complication and Learning Curves for Video-Assisted Thoracic Surgery Lobectomy. Thorac Surg Clin 2008;18:275-80. [Crossref] [PubMed]
- Konge L, Petersen RH, Hansen HJ, et al. No extensive experience in open procedure is needed to learn lobectomy by video-assisted surgery. Interact Cardiovasc Thorac Surg 2012;15:961-5. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Video-Assisted Thoracic Surgical Lobectomy for Lung Cancer: Description of learning Curve. J Laparoendosc Adv Surg Tech A 2017;27:696-703. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK. Competence versus mastery. The time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Shimada Y. Future strategy of surgical simulations in video-assisted thoracic surgery. Video-assist Thoracic surg 2018;3:31. [Crossref]
- Stupnik T, Stork T. Training of video-assisted thoracoscopic surgery lobectomy. The role of simulator. Shanghai Chest 2018;2:52. [Crossref]
- Tedde ML, Filho FB, Belmonte E de A, et al. Video-assisted thoracoscopic surgery in swine: an animal model for thoracoscopic lobectomy training. Interact Cardiovasc Thorac Surg 2015;21:224-30. [Crossref] [PubMed]
- Jimenez M, Gomez-Hernandez MT. Teaching video-assisted thoracic surgery lobectomy-using an ex vivo simulation model. J Vis Surg 2017;3:34. [Crossref] [PubMed]
- Jensen K, Bjerrum F, Hansen HJ, et al. A new possibility in thoracoscopic virtual simulation training: development and testing of a noval virtual reality simulator for video-assisted thoracoscopic surgery lobectomy. Interact Cardiovasc Thorac Surg 2015;21:420-6. [Crossref] [PubMed]
- Bjurström JM, Konge L, Lehnert P, et al. Simulation-based training for thoracoscopy. Simul Healthc 2013;8:317-23. [Crossref] [PubMed]
- Jensen K, Bjerrum F, Hansen HJ, et al. Using virtual reality simulation to assess competence in video-assisted thoracoscopic surgery(VATS) lobectomy. Surg Endosc 2017;31:2520-8. [Crossref] [PubMed]
- Konge L, Lehnert P, Hansen HJ, et al. Reliable and valid assessment of performance in thoracoscopy. Surg Endosc 2012;26:1624-8. [Crossref] [PubMed]
- Jensen K, Petersen RH, Hansen HJ, et al. A novel assessment tool for evaluating competence in video-assisted thoracoscopic surgery lobectomy. Surg Endosc 2018;32:4173-82. [Crossref] [PubMed]
- Petersen RH, Gjeraa K, Jensen K, et al. Assessment of comptence in Video Assisted Thoracoscopic Surgery (VATS) lobectomy: A Danish nationwide study. J Thorac Cardiovasc Surg 2018;156:1717-22. [Crossref] [PubMed]
- Kohls-Gatzoulis JA, Regehr G, Hutchisen C. Teaching cognitive skills improves learning in surgical skills courses: a blinded, prospective randomized study. Can J Surg 2004;47:277-83. [PubMed]
- Savran MM, Hansen HJ, Petersen RH, et al. Development and validation of a theoretical test of a proficiency for video-assisted thoracoscopic surgery (VATS) lobectomy. Surg Endosc 2015;29:2598-604. [Crossref] [PubMed]
- Gjeraa K, Mundt AS, Spanager L, et al. Important Non-Technical Skills in Video-Assisted Thoracoscopic Surgery Lobectomy. Team Perspectives. Ann Thorac Surg 2017;104:329-35. [Crossref] [PubMed]
- Amore D, Curcio C. Steps in the development of a VATS lobectomy program. J Vis Surg 2017;3:104. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning thoracoscopic lobectomy. Eur J Cardiothorac Surg 2010;37:516-20. [Crossref] [PubMed]
- Konge L, Petersen RH, Ringsted C. Developing competency in video-assisted thoracic surgery(VATS) lobectomy. J Thorac Dis 2018;10:S2025-8. [Crossref] [PubMed]
- Ferguson J, Walker W. Developing a VATS lobectomy program-can VATS lobectomy be taught? Eur J Cardiothorac Surg 2006;29:806-9. [Crossref] [PubMed]
- Wan IY, Thung KH, Hsin MK, et al. Video-assisted thoracic surgery major lung resection can be safely taught to trainees. Ann Thorac Surg 2008;85:416-9. [Crossref] [PubMed]
- Reed MF, Lucia MW, Starnes SL, et al. Thoracoscopic lobectomy: Introduction of a new technique into a thoracic surgery training program. J Thorac Cardiovasc Surg 2008;136:376-81. [Crossref] [PubMed]
- Sandri A, Filosso PL, Lausi PL, et al. VATS lobectomy program: The trainee perspective. J Thorac Dis 2016;8:S427-30. [Crossref] [PubMed]
- Billè A, Okiror L, Harrison-Phipps K, et al. Does Previous Surgical Training Impact the Learning Curve in Video-Assisted Thoracic Surgery Lobectomy for Trainees? Thorac cardiovasc Surg 2016;64:343-7. [PubMed]
- Ra YJ, Ahn HY, Kim MS. Learning Curve of a Young Surgeons Video-assisted Thoracic Surgery Lobectomy during His First Year Experience in Newly Established Institution. Korean J Thorac Cardiovasc Surg 2012;45:166-70. [Crossref] [PubMed]
Cite this article as: Haidari TA, Konge L, Petersen RH. Training and precision surgery in VATS lobectomy. Precis Cancer Med 2019;2:20.