
非小细胞肺癌中生物标志物的作用和发展空间
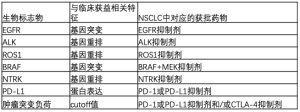
近些年来,临床医生会根据肿瘤组织的分子检测结果来制定晚期肿瘤患者的治疗方案。值得注意的是,癌症治疗的相关文献中充斥着许多未经充分验证的预后和/或预测生物标记物[1,2]。表1中列出经多中心前瞻性临床研究验证的最常见的NSCLC生物标记物。通常为已知肿瘤驱动基因的体细胞突变或基因重排。
Full table
这些生物标志物与临床获益终点[例如总生存期(overall survival,OS)获益]的关联更为有限。患者更加关注类似OS、生活质量改善等指标,而不是医生眼里诸如无进展生存期(progression-free survival,PFS)或客观缓解率(overall response rate,ORR)的重要指标[3]。尽管PFS及ORR通常是药物获得监管部门批准的标准,但通常与OS获益并无关联[4,5]。并且,与OS相比,药物上市前美其名曰节省大量时间的替代性终点的作用似乎被夸大[6]。由于鲜有已获批抗癌药物因缺乏疗效而被监管部门撤回的现象,夸大作用这一点显得尤为突出。
应用NGS平台可检测数百种生物标记物,因其成本效益优于小panel检测而受到吹捧[7-10]。然而,每次NGS的费用通常高达数千美元,且不包括组织/血液采集成本以及相关设备费用,这些费用报价依然非常高。由于NGS超出常规检测的优越性缺乏随机临床试验的验证,对其应用日益具有挑战性[8,11]。
2017年,美国食品药品管理局(Food & Drug Administration,FDA)批准/授权两项肿瘤组织分子检测平台[12]。表2列出目前可用于检测肿瘤组织和血液的NGS平台。已有多篇文章对NGS平台间的一致性进行了评估。但其中很少关注到DNA水平变化以及同期收集的配对的肿瘤和/或血液样本[13,14]。同一患者配对样本收集间隔时间越长(数月/数年),克隆及突变谱发生变化的几率越高[15,16]。

Full table
目前,有许多研究正在探索与诊疗相关的生物标志物,包括非编码RNA、外泌体、微生物菌群等。除肿瘤组织和血样外,其他检测标本包括尿液、支气管灌洗液、粪便及唾液等。发现及验证生物标记物的局限性包括研究时标本易获得但并无预先研究计划、应用未经验证的替代性终点、样本量不足、样本质量堪忧、和/或无法重复独立队列中的研究结果[2]。
NSCLC中两项与临床获益最常见的预测指标为PD-L1免疫组化(immonutherapy,IHC)染色及肿瘤突变负荷(tumor mutation burden,TMB)。KEYNOTE-024(KN-024)研究中检查点抑制剂pembrolizuma对比化疗在PD-L1 IHC≥50%的一线晚期NSCLC患者中达到了其总生存期(overall survival,OS)及无进展生存期(progression free survival,PFS)[17]。虽然IHC染色有多种方法及判定标准,但是最常用的是KN-24研究中应用的22C3 pharmDx检测,后续前瞻性研究结果亦支持其在NSCLC中的应用。由于数据表明体系突变变可能导致新肽表位增加,增强肿瘤免疫原性,因此TMB被认为是疗效预测标志物。不同检测平台间的不一致性、阈临界值的可重复性及检测的周期长短都成为制约TMB广泛应用的因素[18]。目前,TMB在NSCLC中对OS获益的预测作用并未得到认证。
尽管文献报道NSCLC的诊疗方法层出不穷、前景光明,但绝大多数方法经严格验证后证明并不成功,这个领域仍有很大改进空间。无论是将现有药物和既往确定的生物标志物靶点联系起来,还是将来未知的标志物与未开发的抗肿瘤药物的联系起来,都有很长的路要走。
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors Alfredo Addeo and Giuseppe Banna for the series “Non-Small Cell Lung Cancer (NSCLC)” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.05.05). The series “Non-Small Cell Lung Cancer (NSCLC)” was commissioned by the editorial office without any funding or sponsorship. GJW reported receiving grants (to his institution) from GlaxoSmithKline, Pfizer, Incyte, Asana, Macrogenics, and Agenus; receiving consulting fees from and holding ownership interest (service provider units) in Circulogene; receiving consulting fees from Paradigm Diagnostics, Angiex, GLG Council, Guidepoint Global, Imaging Endpoints II, and IBEX Medical Analytics; receiving honoraria from Igynta, Pfizer, and IDEA Pharma; receiving travel and accommodation expenses from Cambridge Healthtech Institute, Tesaro, and GlaxoSmithKline; and holding a patent (PCT/ US2011/020612) related to small cell lung cancer. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Poste G. Bring on the biomarkers. Nature 2011;469:156-7. [Crossref] [PubMed]
- Goossens N, Nakagawa S, Sun X, et al. Cancer biomarker discovery and validation. Transl Cancer Res 2015;4:256-69. [PubMed]
- Hwang TJ, Gyawali B. Association between progression-free survival and patients' quality of life in cancer clinical trials. Int J Cancer 2019;144:1746-51. [Crossref] [PubMed]
- Gyawali B, Hey SP, Kesselheim AS. A Comparison of Response Patterns for Progression-Free Survival and Overall Survival Following Treatment for Cancer With PD-1 Inhibitors: A Meta-analysis of Correlation and Differences in Effect Sizes. JAMA Netw Open 2018;1:e180416. [Crossref] [PubMed]
- Haslam A, Hey SP, Gill J, et al. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur J Cancer 2019;106:196-211. [Crossref] [PubMed]
- Chen EY, Joshi SK, Tran A, et al. Estimation of Study Time Reduction Using Surrogate End Points Rather Than Overall Survival in Oncology Clinical Trials. JAMA Intern Med 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Tan O, Shrestha R, Cunich M, et al. Application of next-generation sequencing to improve cancer management: A review of the clinical effectiveness and cost-effectiveness. Clin Genet 2018;93:533-44. [Crossref] [PubMed]
- Regier DA, Weymann D, Buchanan J, et al. Valuation of Health and Nonhealth Outcomes from Next-Generation Sequencing: Approaches, Challenges, and Solutions. Value Health 2018;21:1043-7. [Crossref] [PubMed]
- Doble B, John T, Thomas D, et al. Cost-effectiveness of precision medicine in the fourth-line treatment of metastatic lung adenocarcinoma: An early decision analytic model of multiplex targeted sequencing. Lung Cancer 2017;107:22-35. [Crossref] [PubMed]
- Marino P, Touzani R, Perrier L, et al. Cost of cancer diagnosis using next-generation sequencing targeted gene panels in routine practice: a nationwide French study. Eur J Hum Genet 2018;26:314-23. [Crossref] [PubMed]
- Prasad V. Why the US Centers for Medicare and Medicaid Services (CMS) should have required a randomized trial of Foundation Medicine (F1CDx) before paying for it. Ann Oncol 2018;29:298-300. [Crossref] [PubMed]
- Goldberg KB, Blumenthal GM, Pazdur R. The First Year of the Food and Drug Administration Oncology Center of Excellence: Landmark Approvals in a Dynamic Regulatory Environment. Cancer J 2018;24:131-5. [Crossref] [PubMed]
- Heeke S, Hofman V, Long-Mira E, et al. Use of the Ion PGM and the GeneReader NGS Systems in Daily Routine Practice for Advanced Lung Adenocarcinoma Patients: A Practical Point of View Reporting a Comparative Study and Assessment of 90 Patients. Cancers (Basel) 2018; [Crossref] [PubMed]
- Chen K, Zhang J, Guan T, et al. Comparison of plasma to tissue DNA mutations in surgical patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;154:1123-1131.e2. [Crossref] [PubMed]
- Toor OM, Ahmed Z, Bahaj W, et al. Correlation of Somatic Genomic Alterations Between Tissue Genomics and ctDNA Employing Next-Generation Sequencing: Analysis of Lung and Gastrointestinal Cancers. Mol Cancer Ther 2018;17:1123-32. [Crossref] [PubMed]
- Chae YK, Davis AA, Carneiro BA, et al. Concordance between genomic alterations assessed by next-generation sequencing in tumor tissue or circulating cell-free DNA. Oncotarget 2016;7:65364-73. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Addeo A, Weiss GJ. Measuring tumor mutation burden in cell-free DNA: advantages and limits. Transl Lung Cancer Res 2019; [Epub ahead of print].

缪延栋
兰州大学在读医学博士研究生。主要研究方向为胃肠道肿瘤的基础与临床研究。近3年来先后以第一作者、共同第一作者或通讯作者的身份在SCI收录期刊发表文章13篇,单篇最高引用14次,累计IF 40余分。目前担任10余本SCI期刊审稿人。兼任World Journal of Gastrointestinal Surgery的学术编辑。(更新时间:2021/8/15)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Weiss GJ. Role and room for biomarkers in non-small cell lung cancer. Precis Cancer Med 2019;2:18.


