Custom-made prosthesis in thoracic surgery
Introduction
After wide excision of primary or metastatic chest wall tumors, reconstruction is needed not only to preserve cosmesis but to prevent respiratory compromise and to afford some protection to underlying organs. The most common materials used for reconstruction are synthetic meshes, methyl methacrylate patches and more recently titanium implants (1,2). Unfortunately, all these materials share similar inconveniences: resection margins are not prefixed before operation but subjectively fixed according to the findings and impressions during surgery so it is usual that the implant needs to be molded or cut thus leading to inaccurate fitting; fixation systems are mechanically unstable what increases the risk for device migration and finally, these prostheses are flat and rigid, being able to cause erosion of neighboring tissues, stiffness, pain, respiratory impairment and bad cosmetic results.
In an effort to solve these problems associated with traditional reconstruction methods and under the promise of better functional and cosmetic results, interest has been renewed for more customized solutions as those provided by additive manufacturing (AM), commonly known as tridimensional (3D) printing.
What is AM?
AM is a process of joining materials to make objects from 3D model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies. Since its inception in the aerospace and automotive industries, its applications in medicine are increasing its relevance during the last number of years.
The AM workflow starts with a creative design, followed by a manufacturing time and a post processing stage to end with a validation and quality process.
Design and manufacturing considerations
Next to validation and quality process, creative design is the most important phase of AM because close collaboration between the designers and clinicians is needed to ensure the design meets the desired specifications from a clinical, mechanical, and manufacturing point of view. Besides this, an initial assessment including possible technical or manufacturing handicaps should be done taking in consideration technologies and materials to be used and the desired final purpose of the medical device.
The design stage starts with patient’s own medical images in digital format. The image source can vary depending on the available equipment, being DICOM one of the most common formats for 3D file exchange. Once a 3D model is virtually created via dedicated software, the type of AM technique is chosen, the printing parameters are set and the whole process is revised to ensure that all software and systems have been qualified for the manufacturing of medical devices and are compliant with medical device manufacturing quality standards.
Regarding the materials to be used for printing, biocompatible and osteoinductive materials are preferred for thoracic prostheses. Briefly, these materials can be classified in different families of polymers, ceramics, metals and composites (3). Polymers have been one of the most used materials in 3D printing applications such as cranial implants (4) or tracheomalacia splints (5) in the form of bioabsorbable polymers such as poly-ε-caprolactone (PCL) (6,7), Poly(l-lactide) (PLLA), poly(L-lactide-co-glycolide) (PLAGA), or polyethylene (PE) and recently as polymer-ceramic blends from poly(l-lactide)/carbonated hydroxyapatite composites or Poly-e-caprolactone/hydroxyapatite scaffolds (8). Metals and their alloys (most commonly titanium or stainless steel) are used for load-bearing implants (e.g., hip or knee) due to their mechanical reliability, strength and stiffness; in thoracic surgery, their toughness and impact resistance have made them especially attractive for the manufacture of thoracic wall prostheses. Finally, ceramics have excellent biocompatibility and they are usually employed to coat the metallic core structures of prosthesis, thus the ceramic provides the hardness and wear resistance while the metallic core provides toughness and high strength for load bearing applications (9). Examples of different printable ceramics are alumina, zirconia, silicon nitride or tricalcium phosphate.
Post-processing stage
Once the component has been manufactured, the procedure continues with post-processing stage, where the printed device is separated from the plate and supports on which is printed and further steps are executed, such as machining (roughing and polishing), heat treatment or cleaning and sterilization.
Validation and quality process
A printed device has a good quality when the objectives for which it has been designed and manufactured are perfectly fulfilled or in other words, when its design, material characteristics and properties correspond exactly with the ones planned.
Not all AM techniques can be used to manufacture implantable devices, since some do not meet the minimum mechanical, physical or chemical requirements for this. The manufacturing inputs for every AM technique (parameters, process steps and raw materials) are different and may even vary within each of them. Moreover, printing materials can suffer very variable physical and chemical changes depending on the type of printing procedure thus leading to radically different outputs (final devices) with different levels of quality. This means that quality achievement strongly depends on the knowledge around how each single detail of the manufacturing process affects the final results. Although most of the times it is possible to achieve adequate quality only by exhaustively control of the manufacturing process, in other cases further validation steps should be considered, verifying through specific inspection and testing the final quality of the device. In addition and for patient-matched devices, the final product is different in each case (one patient, one custom-made implant) so the quality certification is around the process and not around the product itself. Therefore, when accessing to AM services for the manufacturing of implantable devices, a clinician must be fully sure about the expertise of the company in the field and the existence of quality control systems specifically validated for the manufacture of medical devices, such as ISO 13485.
AM applications in healthcare
As with any new medical technology, research efforts in AM are now focused in establishing its utility in various clinical settings. This technology is a rising trend in medicine as proved by the existence of dedicated radiology printing labs in very relevant hospitals such as The Mayo Clinic. In these facilities, investigators create on-demand medical devices with a broad range of biological and physical properties that can be paired with medical imaging (computed tomography magnetic resonance or 3-dimensional ultrasound) to be tailored to an individual patient’s anatomy.
AM applications in surgery
Most of the applications of 3D printing in surgery are focused on these four categories: surgical 3D models, surgical tools and templates, tissue engineering and implants:
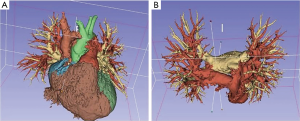
- Models: 3D printed models have been successfully tested in many surgical disciplines for pre-operative planning, counselling with patients, education of students/residents and surgical training. They are highly accurate reproductions that allow for tactile and 3D inspection of the tissues, what help the comprehension of anatomical details in a more effective way than conventional 2D imaging and 3D virtual models, giving the surgeon the ability to plan out accurately what must be done during the operation. Examples of these models are printed aortic devices for vascular surgery, aneurysm model for endovascular repair, cardiac simulation for planning of tumor resections and repair of congenital defects, neurosurgical navigation training and planning of tumor resection and treatment of trauma injuries in orthopedic surgery (10). Within thoracic surgery field, some relevant centers such as The Mayo Clinic has been using them since 2006 for educational and clinical purposes (11) and now its use is very common, being a basic tool also in our center (Figures 1 and 2
). - Surgical tools and templates: AM is also employed for adaptation or creation of new surgical tools that improve access to the surgical field, simplifying the operation and improving its outcomes. Patient-specific surgical templates are especially interesting to guide a surgical instrument for precise handling where exact cutting or drilling is required as frequently occurs in maxillofacial or orthopedic surgeries; in some thoracic interventions, these templates has also been used as the way to allow intraoperative precise setting of resection margins for a perfect implant fitting (12).
- Tissue engineering (13): although only some preliminary steps are being taken, the last promising application for AM in surgery is tissue engineering. A 3D printer can bind new porous biomaterials to form complex ceramic scaffolds that encourage the growth of bone in any shape (14). An even more specific approach would be two-phase systems (loaded nanoparticles embedded into printed scaffolds seeded with stem cells (15,16), since some of them offer promising results even in cases of full-thickness chest wall defects (17). Nowadays, the use of tissue engineered printed devices in thoracic surgery remain limited to some experimental, non-clinical cases.
- Implants: the manufacture of custom made 3D printed implants is clearly the main utility of 3D printing in surgery. Specialties such as maxillofacial surgery (fixation plates and screws, mandibular prostheses, polyethylene plates for orbital reconstruction…), neurosurgery (cranioplasty plates for reconstructing skull defects) or orthopedics, (bony prostheses for reconstruction after tumor resection, customized external fixators for treating fractures, cervical spine reconstruction…) are now common users of these technologies.
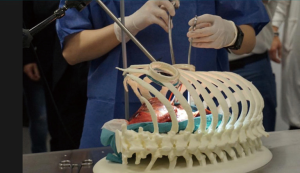
This aspect of AM is also the most developed within our specialty to have various clinical applications: from tracheal splints to treat tracheomalacia (5) going through personalized bronchial stents (18) until you get to custom made sternocostal implants, where most of the efforts are now concentrated especially for reconstruction in complex cases or in areas with more specific anatomical or functional requirements such as the sternal area (12,19-21) (Figure 3). Step by step, the indications for the use of these devices are being extended to other areas of the chest wall in an attempt to solve more specific problems such as, for example, reconstruction in the pediatric population (22,23).

A day to day technology in thoracic surgery?
Despite the great advances made in the field of 3D printing, the use of custom –made thoracic prostheses has been quite limited. To start with, many thoracic surgeons are reluctant to this new technology and consider them as “the new, expensive and unnecessary way to do things as they have always been done”. This way of thinking is usually reinforced by the fact that there are many experimental studies on 3D printing but related clinical studies are scarce, heterogeneous and reports inconsistent short and mid-term outcomes. Given this framework, is clear that if we want to solve the problem of translational evidence (translation of experimental data into daily clinical experience) we need new rules for this game and suggestions for future prototyping could be grouped in two different areas:
- Technical and functional issues: there is a need for novel techniques and materials suitable for 3D printing that lead to reduce prostheses weight and rigidity, develope easier and more secure anchoring systems and fine tune of resection margins measurement. Most of these improvements depend in fact on materials engineering, software or industrial design thus are beyond our scope, but collaboration with dedicated 3D printing engineers is crucial in order to guide their efforts towards clinically-relevant objectives to be initially tested in mechanical assays followed by experimental studies on animal models ideally linked to phase I clinical trials.
- Macroscopical function of a 3D prosthesis is linked to its external morphology or design. Beyond merely aesthetic results, this aspect is important for their articulation within the chest wall not to impair its normal function. All of actually released devices (19-21) claim to improve functional outcomes after chest wall reconstruction but unfortunately, this affirmation is far away to be truth because these prostheses are capable to flex and distend but the real dynamics of the thoracic cage is much more complex .Specific criteria for motion range evaluation of these prostheses are neither stated nor applied (24) although new biomechanical simulation models of thorax deformation (finite element approach) (25) promise to revolutionize functional design and some recent papers about in vivo assessment of post implantation function via optoelectronic plethysmography (26) can help in this aspect too.
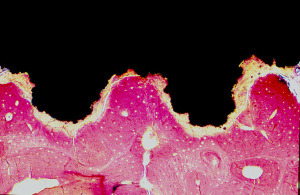
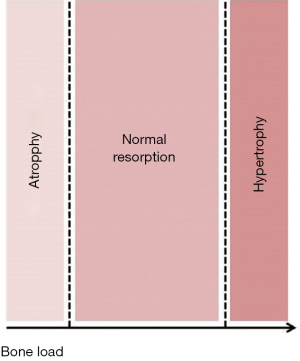
- There is also a microscopical function based on the prosthesis properties at micro and nanoscale such as chemical composition of the material of which is made, grain size and shape, microporosity or roughness (3) at the intimate contact surface between the host and the implant (interface). This interface is fully customizable to achieve better integration with the host tissue (adequate anchoring at the time of placement, absence of mobility after healing) (Figure 4) and balanced adaptive remodeling (absence of sclerosis or osteoporosis in the host bone as response to implantation) (Figure 5). Equally to technical aspects, improvements in both integration and remodeling largely depend on experimental, cooperative studies and as much for technical as for functional issues, the final goal should be full standardization, that is, design and manufacturing process is fully standardized for all type of thoracic prostheses (costal, sternal, clavicular…), so that only small modifications would have to be made to adapt it to each particular case.
- Logistic issues: as stated by Okereke (24), there is a real concern on the reduction of manufacturing costs and time to further increase the accessibility of 3D printing. The ability to quickly create a custom implant that is safe to use in the patient and comparatively low cost is the key factor here and as previously said, it depends on technical and functional improvements to achieve standardized manufacturing, what would allow us to combine the best of customization with production on an industrial scale, favoring the alternative use of these implants with indications similar to those of other reconstruction techniques.
Although 3D printing offers great potential for its application in surgery, there are a few significant issues to overcome before it can be considered as a common technology (27). Some of these clinical issues has already been previously mentioned (weight, tissue integration…) while many other technical aspects such as the printing speed and resolution of the printers, versatility and diversity in printable biomaterials, quality control or regulatory hurdles should be addressed before these 3D printed devices may be considered common use on a day to day basis.
Conclusions
AM technology has shown the potential in manufacturing new designs that could not be dreamt before, allowing new applications to be done every day. However, it is still important to consider its limitations of the process and rules because 3D printing processes are complex manufacturing technologies with a wide number of variables involved in the process and therefore building experience around the process has become vital.
There is no doubt that 3D printing has an immense potential in health care in general and in thoracic surgery in particular. It is crucial to understand that we are immersed in a new era in which the borders between disciplines such as industrial engineering, chemical engineering, biomedicine or clinical medicine are becoming blurred. Clearly, the future of our specialty goes through multidisciplinary groups, with this cooperation leading lead us to the development of new customized prostheses increasingly functional, cheap and sufficiently standardized to be able to be produced on an industrial scale without losing its personalized character. However, as in any other field of innovation, great advances will require some patience and a good deal of investment in order to develop enough well designed experimental and phase I clinical studies to achieve enough translational clinical evidence to boost broad dissemination of medical 3D printing at an affordable cost.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Marcelo F. Jimenez for the series “Precision Surgery for Lung Cancer” published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.04.02). The series “Precision Surgery for Lung Cancer” was commissioned by the editorial office without any funding or sponsorship. AME currently works as Director of Technology for Steripack Group. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ng CS. Recent and Future Developments in Chest Wall Reconstruction. Semin Thorac Cardiovasc Surg 2015;27:234-9. [Crossref] [PubMed]
- Sandler G, Hayes-Jordan A. Chest wall reconstruction after tumor resection. Semin Pediatr Surg 2018;27:200-6. [Crossref] [PubMed]
- Barradas AM, Yuan H, van Blitterswijk CA, et al. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 2011;21:407-29. [Crossref] [PubMed]
- Bonda DJ, Manjila S, Selman WR, et al. The Recent Revolution in the Design and Manufacture of Cranial Implants. Neurosurgery 2015;77:814-24. [Crossref] [PubMed]
- Morrison RJ, Sengupta S, Flanangan CL, et al. Treatment of Severe Acquired Tracheomalacia With a Patient-Specific, 3D-Printed, Permanent Tracheal Splint. JAMA Otolaryngol Head Neck Surg 2017;143:523. [Crossref] [PubMed]
- Coelho PG, Hollister SJ, Flanagan CL, et al. Bioresorbable scaffolds for bone tissue engineering: Optimal design, fabrication, mechanical testing and scale-size effects analysis. Med Eng Phys 2015;37:287-96. [Crossref] [PubMed]
- Partee B, Hollister SJ, Das S. Selective Laser Sintering Process Optimization for Layered Manufacturing of CAPA[sup ®] 6501 Polycaprolactone Bone Tissue Engineering Scaffolds. J Manuf Sci Eng 2006;128:531. [Crossref]
- Eosoly S, Brabazon D, Lohfeld S, et al. Selective laser sintering of hydroxyapatite/poly-ε-caprolactone scaffolds. Acta Biomater 2010;6:2511-7. [Crossref] [PubMed]
- Vlasov AS, Karabanova TA. Ceramics and Medicine Glass and Ceramics 1993;50:398-401. (Review). [Crossref]
- Shilo D, Emodi O, Blanc O, et al. Printing the Future.Updates in 3D Printing for Surgical Applications. Rambam Maimonides Med J 2018;9:e0020. [Crossref] [PubMed]
- Blackmon S. 3D Printing in Thoracic, Congenital, and Cardiac Surgery. Available online: https://www.ctsnet.org/article/3d-printing-thoracic-congenital-and-cardiac-surgery.
- Aranda JL, Jimenez MF, Rodriguez M, et al. Tridimensional titanium-printed custom-made prosthesis for sternocostal reconstruction. Eur J Cardiothorac Surg 2015;48:e92-4. [Crossref] [PubMed]
- Novoa NM, Aranda Alcaide JL, Gomez Hernández MT, et al. Chest wall reconstruction: yesterday, today and the future. Shanghai Chest 2019;3:15. [Crossref]
- Daly AC, Cunniffe GM, Sathy BN, et al. 3D Bioprinting of Developmentally Inspired Templates for Whole Bone Organ Engineering. Adv Healthc Mater 2016;5:2353-62. [Crossref] [PubMed]
- Michalski MH, Ross JS. The shape of things to come: 3D printing in medicine. JAMA 2014;312:2213-4. [Crossref] [PubMed]
- De Witte TM, Fratila-Apachitei LE, Zadpoor AA, et al. Bone tissue engineering via growth factor delivery: from scaffolds to complex matrices. Regen Biomater 2018;5:197-211. [Crossref] [PubMed]
- Zhang Y, Fang S, Dai J, et al. Experimental study of ASCs combined with POC-PLA patch for the reconstruction of full-thickness chest wall defects. PLoS One 2017;12:e0182971. [Crossref] [PubMed]
- Guibert N, Didier A, Moreno B, et al. Treatment of Post-transplant Complex Airway Stenosis with a Three-Dimensional, Computer-assisted Customized Airway Stent. Am J Respir Crit Care Med 2017;195:e31-3. [Crossref] [PubMed]
- Cano JR, Escobar FH, Alonso DP, et al. Reconstruction of the anterior chest wall with a 3-dimensionally printed biodynamic prosthesis. J Thorac Cardiovasc Surg. 2018;155:e59-60. [Crossref] [PubMed]
- Aragón J, Pérez Méndez I. Dynamic 3D printed titanium copy prosthesis: a novel design for large chest wall resection and reconstruction. J Thorac Dis 2016;8:E385-9. [Crossref] [PubMed]
- Moradiellos J, Amor S, Córdoba M, et al. Functional Chest Wall Reconstruction With a Biomechanical Three-Dimensionally Printed Implant. Ann Thorac Surg 2017;103:e389-91. [Crossref] [PubMed]
- Sandler G, Hayes-Jordan A. Chest wall reconstruction after tumor resection. Semin Pediatr Surg 2018;27:200-6. [Crossref] [PubMed]
- Makarawo TP, Reynolds RA, Cullen ML. Polylactide bioabsorbable struts for chest wall reconstruction in a pediatric patient. Ann Thorac Surg 2015;99:689-91. [Crossref] [PubMed]
- Okereke IC. Is 3-dimensional printing the right fit for your reconstruction? J Thorac Cardiovasc Surg 2018;155:e61-2. [Crossref] [PubMed]
- Zhang G, Chen X, Ohgi J, et al. Biomechanical simulation of thorax deformation using finite element approach. Biomed Eng Online 2016;15:18. [Crossref] [PubMed]
- Oswald N, Senanayake E, Naidu B, et al. Chest Wall Mechanics In Vivo With a New Custom-Made Three-Dimensional-Printed Sternal Prosthesis. Ann Thorac Surg 2018;105:1272-6. [Crossref] [PubMed]
- Roopavath UK, Kalaskar DM. Introduction to 3D printing in Medicine, In: Kalaskar DM, editor. 3D Printing In Medicine. Woodhead Publishing, 2017
Cite this article as: Aranda JL, Espiago AM. Custom-made prosthesis in thoracic surgery. Precis Cancer Med 2019;2:15.