
Disease progression in non-small cell lung cancer on immune-checkpoint inhibition, what are the options?
Introduction
Immune-checkpoint inhibitors are moving into first-line therapy of advanced non-small cell lung cancer (NSCLC) for tumours without driver-mutation. For high expressers of PD-L1, i.e., a tumour proportional score (TPS) of 50% or higher pembrolizumab monotherapy demonstrated in comparison to doublet chemotherapy an advantage regarding toxicity, progression-free survival and overall survival (1,2). For all-commers regarding PD-L1-expression the combination of pembrolizumab with pemetrexed and cis-/carboplatin was superior in comparison to the chemotherapy alone group in non-squamous NSCLC (3). Atezolizumab plus Paclitaxel, Carboplatin and Bevacizumab was also superior in comparison to the three-drug regimen (4). In squamous cell cancer atezolizumab plus carboplatin plus nab-paclitaxel improves progression-free and overall survival (5). This is also true for pembrolizumab plus paclitaxel or nab-paclitaxel and carboplatin (6). In NSCLC with high mutational burden the combination of PD-1- and CTLA4-inhibition showed a benefit over chemotherapy irrespective the PD-L1 expression (7)). Furthermore a consolidation therapy with durvalumab after simultaneous chemoradiotherapy had relevant advantage in survival (8,9). We have good evidence that PD-1-/PD-L1 inhibition works after first-line chemotherapy (10-13), but there are very few and almost no prospective data how we should proceed with refractory or progressive tumours having first-line therapy with immune-checkpoint inhibition. We present therefore in the following strategies according to the various first-line settings how we can treat after first-line immune check-point inhibition. These strategies have of course to be checked and confirmed with prospective data in registries and clinical trials.
Disease progression after stopping immune check-point inhibition due to toxicity or after an extended period of treatment
If there is disease progression after stopping immune check-point inhibition due to toxicity re-establishing immune check-point inhibition seems to be an option (14). After long-term application of immune check-point inhibition and durable response re-induction at progression seems to be possible (15). This topic will be elaborated in a separate manuscript in this issue of PMC.
Disease progression during PD-1 inhibition as monotherapy
In case of disease progression during PD-1 inhibition as monotherapy a classical chemotherapy doublet can be given (3) and is probably adequate. In the case of only one or few sites of progression local therapies, especially radiotherapy can be added (16,17). Radiation therapy leads to an immunological reaction, which can end in tumour destruction distant to the irradiated area (abscopal effect) (18). In a systematic review of 46 cases between 1969 and 2014 there was a wide variety in the cases and the abscopal effect was described after up to 12 months (19). The abscopal effect was also described after radiotherapy and ipilimumab (20). In a phase II trial pembrolizumab after locally ablative therapy in the oligometastatic setting demonstrated a reasonable progression-free and overall survival (21). The combination with other immune therapies is also an option for clinical trials (22,23). One principle of acquired resistance is the exhaustion of T-cells, which could be overcome by adding an alternative stimulation. One example is the addition of nivolumab after the CTLA4-antibody ipilimumab (24). Also targeting TGF-ß in addition to PD-1 may be an option (25).
Disease progression during or after immune check-point inhibition and chemotherapy (plus bevacizumab)
After the combination of immune check-point inhibition and doublet chemotherapy (plus bevacizumab) probably standard second-line mono-chemotherapy with docetaxel or pemetrexed (in non-squamous NSCLC, if not given already in first-line) is a reasonable choice. Chemotherapy may also be immunogenic and enable further immune check-point inhibition (26). In the second-line setting after doublet chemotherapy the addition of ramucirumab or nintedanib (in non-squamous NSCLC) is of benefit. This seems to be true especially for tumours who relapse or progress early (27-29). Probably this is also true after immune check-point inhibition, even in the third-line setting after chemotherapy and nivolumab (30,31). As described already above also in the setting of mono- or oligo-progression with immune check-point inhibition and chemotherapy (plus bevacizumab) locally ablative therapies can be useful. If available also further immune therapy approaches can be tested in clinical trials (32). Examples are pegilodecakin (IL-10) (33), entinostat (HDAC inhibitor) (34), toll-like receptor 9 agonists, e.g., lefitolimod (35), Adenosine-antagonists (36,37) and adoptive cell transfer using tumour infiltrating lymphocytes (38).
Disease progression during or after a combination of PD-1- and CTLA4-inhibition
If in first-line a combination of PD-1- and CTLA4-inhibition was used, classical doublet chemotherapy is probably the preferred option. Alternatively prospective clinical trials can examine further immune modulating agents. In case of mono- or oligo-progression local treatments can be evaluated—as already described above.
Necessary research
We have good evidence for the second-line setting in NSCLC without driver mutation after a doublet chemotherapy in first-line. As immunotherapy is moving in first-line we have no adequate evidence, how we should handle primary progression or recurrence. The situation can be improved when in all first-line clinical trial the monitoring of second- and third-line therapy is mandatory and includes the treatment regimens, their efficacy and the second and third progression-free survival. Also prospective registries of the patients which get immunotherapy in first-line would be of help. Of course it would be nevertheless necessary to use trial designs where the best approach over the several lines of therapy is tested.
Conclusions
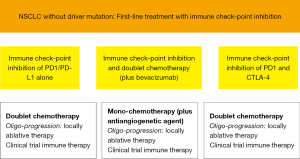
As immunotherapy in NSCLC without driver mutation is moving into first-line we need strategies to treat progression and recurrence. At the moment only recommendations at the level of expert opinions can be given. Our recommendations are outlined in Figure 1. Depending on the regimen in first-line mostly chemotherapy will be applied: as doublet chemotherapy, if only immune check-point inhibition was used, as classical second-line mono chemotherapy after the combination of immune check-point inhibition and doublet chemotherapy (plus bevacizumab) and chemoradiotherapy with immune check-point inhibition. If the progress/recurrence is early the addition of an antiangiogenetic agent (nintedanib, ramucirumab) is probably useful. If there is mono- or oligo-progression only locally ablative therapies may be adequate and foster the efficacy of immune check-point inhibition. Mostly in clinical trials further immune therapy combinations can be applied. Overall we urgently need prospective data to these concepts.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor Nir Peled for the series dedicated to the Congress on Clinical Controversies in Lung Cancer (CCLC 2018) published in Precision Cancer Medicine. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/pcm.2019.03.01). The series dedicated to the Congress on Clinical Controversies in Lung Cancer (CCLC 2018) was commissioned by the editorial office without any funding or sponsorship. RMH reports grants and personal fees from Astra Zeneca, and personal fees from Takeda, Merrimak, Chugai, Abbvie, Novartis, Bayer, BMS, Boehringer Ingelheim, Celgene, Pfizer, Roche, Lilly, Guardant Health, Mologen, MSD, outside the submitted work . The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]
- Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol 2018;36:LBA4. [Crossref]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Socinski MA, Koynov KD, Berard H, et al. LBA65IMpower131: Progression-free survival (PFS) and overall survival (OS) analysis of a randomised phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel in 1L advanced squamous NSCLC. Ann Oncol 2018;29.
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Santini FC, Rizvi H, Plodkowski AJ, et al. Safety and Efficacy of Re-treating with Immunotherapy after Immune-Related Adverse Events in Patients with NSCLC. Cancer Immunol Res 2018;6:1093-9. [Crossref] [PubMed]
- Herbst RS, Monnet I, Novello S, et al. LBA63Long-term survival in patients (pts) with advanced NSCLC in the KEYNOTE-010 study overall and in pts who completed two years of pembrolizumab (pembro). Ann Oncol 2018;29.
- Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:831-9. [Crossref] [PubMed]
- Gide TN, Wilmott JS, Scolyer RA, et al. Primary and Acquired Resistance to Immune Checkpoint Inhibitors in Metastatic Melanoma. Clin Cancer Res 2018;24:1260-70. [Crossref] [PubMed]
- Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39:644-55. [Crossref] [PubMed]
- Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr Probl Cancer 2016;40:25-37. [Crossref] [PubMed]
- Golden EB, Demaria S, Schiff PB, et al. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 2013;1:365-72. [Crossref] [PubMed]
- Bauml J, Misk R, Ciunci C, et al. OA 17.08 - Phase II Study of Pembrolizumab for Oligometastatic Non-Small Cell Lung Cancer (NSCLC) Following Completion of Locally Ablative Therapy (LAT). J Thorac Oncol 2017;12:S1794-5. [Crossref]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Melero I, Berman DM, Aznar MA, et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015;15:457-72. [Crossref] [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [Crossref] [PubMed]
- Barlesi F, Isambert N, Felip E, et al. Initial results from phase 1 trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGF-β, in patients with NSCLC refractory or resistant to prior anti–PD-1/anti–PD-L1 agents. J Immunother Cancer 2017;5:86.
- Galluzzi L, Buque A, Kepp O, et al. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015;28:690-714. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Reck M, Kerr KM, Grohé C, et al. Defining aggressive or early progressing nononcogene-addicted non-small-cell lung cancer: a separate disease entity? Future Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Corral J, Majem M, Rodríguez-Abreu D, et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018;118:9-16. [Crossref] [PubMed]
- Wong D, Goldman J, Gabrail NY, et al. 9PDPEGylated human IL-10 (AM0010) in combination with an anti-PD-1 in advanced NSCLC. Ann Oncol 2017;28:mdx710.
- Hellmann M, Jänne P, Opyrchal M, et al. OA05.01 Efficacy/Safety of Entinostat (ENT) and Pembrolizumab (PEMBRO) in NSCLC Patients Previously Treated with Anti-PD-(L)1 Therapy. J Thorac Oncol 2018;13:S330. [Crossref]
- Thomas M, Ponce-Aix S, Navarro A, et al. Immunotherapeutic maintenance treatment with toll-like receptor 9 agonist lefitolimod in patients with extensive-stage small-cell lung cancer: results from the exploratory, controlled, randomized, international phase II IMPULSE study. Ann Oncol 2018;29:2076-84. [Crossref] [PubMed]
- Mediavilla-Varela M, Castro J, Chiappori A, et al. A Novel Antagonist of the Immune Checkpoint Protein Adenosine A2a Receptor Restores Tumor-Infiltrating Lymphocyte Activity in the Context of the Tumor Microenvironment. Neoplasia 2017;19:530-6. [Crossref] [PubMed]
- Chiappori A, Williams CC, Creelan BC, et al. Phase I/II study of the A2AR antagonist NIR178 (PBF-509), an oral immunotherapy, in patients (pts) with advanced NSCLC. J Clin Oncol 2018;36:9089. [Crossref]
- Creelan B, Teer J, Toloza E, et al. OA05.03 Safety and Clinical Activity of Adoptive Cell Transfer Using Tumor Infiltrating Lymphocytes (TIL) Combined with Nivolumab in NSCLC. J Thorac Oncol 2018;13:S330. [Crossref]
Cite this article as: Huber RM. Disease progression in non-small cell lung cancer on immune-checkpoint inhibition, what are the options? Precis Cancer Med 2019;2:13.

